Axon initial segment Ca2+ channels influence action potential generation and timing
- PMID: 19186168
- PMCID: PMC2730113
- DOI: 10.1016/j.neuron.2008.12.004
Axon initial segment Ca2+ channels influence action potential generation and timing
Abstract
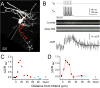
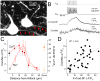
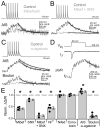
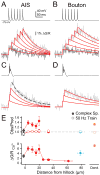
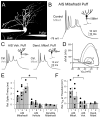
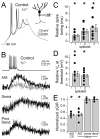
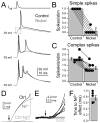
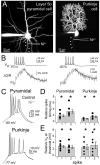
Although action potentials are typically generated in the axon initial segment (AIS), the timing and pattern of action potentials are thought to depend on inward current originating in somatodendritic compartments. Using two-photon imaging, we show that T- and R-type voltage-gated Ca(2+) channels are colocalized with Na(+) channels in the AIS of dorsal cochlear nucleus interneurons and that activation of these Ca(2+) channels is essential to the generation and timing of action potential bursts known as complex spikes. During complex spikes, where Na(+)-mediated spikelets fire atop slower depolarizing conductances, selective block of AIS Ca(2+) channels delays spike timing and raises spike threshold. Furthermore, AIS Ca(2+) channel block can decrease the number of spikelets within a complex spike and can even block single, simple spikes. Similar results were found in cortex and cerebellum. Thus, voltage-gated Ca(2+) channels at the site of spike initiation play a key role in generating and shaping spike bursts.
Figures


References
-
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. - PubMed
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. - PubMed
-
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

