Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function
- PMID: 19187004
- PMCID: PMC2842133
- DOI: 10.1089/ars.2008.2331
Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function
Abstract
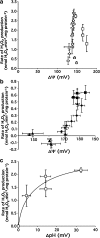
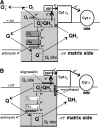
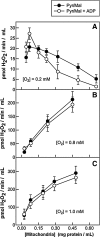
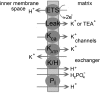
The mitochondrion is a major source of reactive oxygen species (ROS). Superoxide (O(2)(*-)) is generated under specific bioenergetic conditions at several sites within the electron-transport system; most is converted to H(2)O(2) inside and outside the mitochondrial matrix by superoxide dismutases. H(2)O(2) is a major chemical messenger that, in low amounts and with its products, physiologically modulates cell function. The redox state and ROS scavengers largely control the emission (generation scavenging) of O(2)(*-). Cell ischemia, hypoxia, or toxins can result in excess O(2)(*-) production when the redox state is altered and the ROS scavenger systems are overwhelmed. Too much H(2)O(2) can combine with Fe(2+) complexes to form reactive ferryl species (e.g., Fe(IV) = O(*)). In the presence of nitric oxide (NO(*)), O(2)(*-) forms the reactant peroxynitrite (ONOO(-)), and ONOOH-induced nitrosylation of proteins, DNA, and lipids can modify their structure and function. An initial increase in ROS can cause an even greater increase in ROS and allow excess mitochondrial Ca(2+) entry, both of which are factors that induce cell apoptosis and necrosis. Approaches to reduce excess O(2)(*-) emission include selectively boosting the antioxidant capacity, uncoupling of oxidative phosphorylation to reduce generation of O(2)(*-) by inducing proton leak, and reversibly inhibiting electron transport. Mitochondrial cation channels and exchangers function to maintain matrix homeostasis and likely play a role in modulating mitochondrial function, in part by regulating O(2)(*-) generation. Cell-signaling pathways induced physiologically by ROS include effects on thiol groups and disulfide linkages to modify posttranslationally protein structure to activate/inactivate specific kinase/phosphatase pathways. Hypoxia-inducible factors that stimulate a cascade of gene transcription may be mediated physiologically by ROS. Our knowledge of the role played by ROS and their scavenging systems in modulation of cell function and cell death has grown exponentially over the past few years, but we are still limited in how to apply this knowledge to develop its full therapeutic potential.
Figures






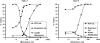







References
-
- Akao M. O'Rourke B. Teshima Y. Seharaseyon J. Marban E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res. 2003;92:186–194. - PubMed
-
- Alberts B. Johnson A. Lewis J. Raff M. Roberts K. Walter P. Molecular biology of the cell. New York: Garland Publishing; 1994.
-
- Aldakkak M. Camara AKS. Beard DA. Dash RK. Huang M. Stowe DF. NS1619 increases potassium influx in the isolated intact mitochondrion in a dose-dependent manner [Abstract] Biophys J. 2007:pos–441.
-
- Aldakkak M. Camara AKS. Patel R. Haumann J. Rhodes SS. Stowe DF. Inactivation of cardiac mitochondrial K/H exchange by quinine exposes matrix acidification and K+ influx by putative Ca2+-dependent K+ channels [Abstract] J Biophysics. 2008:436–pos.
-
- Aldakkak M. Stowe DF. Chen Q. Lesnefsky EJ. Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

