Kinetics of T lymphocyte apoptosis and the cellular immune response in SIVmac239-infected rhesus macaques
- PMID: 19187429
- PMCID: PMC2918415
- DOI: 10.1111/j.1600-0684.2008.00323.x
Kinetics of T lymphocyte apoptosis and the cellular immune response in SIVmac239-infected rhesus macaques
Abstract
Background: Although increased apoptosis is a central feature of AIDS, little is known about its kinetics or relationship to the early host response in acute HIV/SIV infection.
Methods: Ex vivo apoptosis in freshly isolated peripheral blood and lymph node lymphocytes was monitored longitudinally in SIVmac239-infected rhesus macaques by flow-cytometric detection of active caspase-3, cleaved poly (ADP-ribose) polymerase, and fragmented DNA.
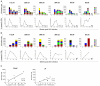
Results: Increased apoptosis of multiple lymphocyte subsets was observed in the first 2 weeks following SIV infection. Apoptosis of CD4+ T lymphocytes was of low magnitude but peaked earlier than other T lymphocyte subsets. A 10- to 36-fold increase in CD8+ T lymphocyte apoptosis coincided temporally with onset of the SIV-specific cellular immune response and enrichment of caspase-3-positive cells within recently proliferating, activated CD8+ T lymphocytes.
Conclusions: The virus-specific T lymphocyte response to primary infection and generalized non-specific immune activation contribute to the pathogenesis of apoptosis in acute SIV infection.
Figures

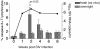
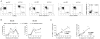

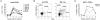
References
-
- Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–7. - PubMed
-
- Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84:1649–61. - PubMed
-
- Anonymous . Guide for care and use of laboratory animals. Institute of Laboratory Animal Resources, National Council; Washington, D.C.: 1996. pp. 86–123.
-
- Appay V, Papagno L, Spina CA, Hansasuta P, King A, Jones L, Ogg GS, Little S, McMichael AJ, Richman DD, Rowland-Jones SL. Dynamics of T cell responses in HIV infection. J Immunol. 2002;168:3660–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

