Estradiol induces physical association of neuronal nitric oxide synthase with NMDA receptor and promotes nitric oxide formation via estrogen receptor activation in primary neuronal cultures
- PMID: 19187438
- PMCID: PMC2743827
- DOI: 10.1111/j.1471-4159.2009.05949.x
Estradiol induces physical association of neuronal nitric oxide synthase with NMDA receptor and promotes nitric oxide formation via estrogen receptor activation in primary neuronal cultures
Abstract
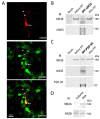
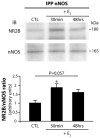
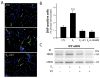
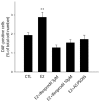
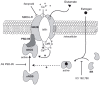
Estrogens and nitric oxide (NO) exert wide-ranging effects on brain function. Recent evidence suggested that one important mechanism for the regulation of NO production may reside in the differential coupling of the calcium-activated neuronal NO synthase (nNOS) to glutamate NMDA receptor channels harboring NR2B subunits by the scaffolding protein post-synaptic density-95 (PSD-95), and that estrogens promote the formation of this ternary complex. Here, we demonstrate that 30-min estradiol-treatment triggers the production of NO by physically and functionally coupling NMDA receptors to nNOS in primary neurons of the rat preoptic region in vitro. The ability of estradiol to activate neuronal NO signaling in preoptic neurons and to promote changes in protein-protein interactions is blocked by ICI 182,780, an estrogen receptor antagonist. In addition, blockade of NMDA receptor NR2B subunit activity with ifenprodil or disruption of PSD-95 synthesis in preoptic neurons by treatment with an anti-sense oligodeoxynucleotide inhibited the estradiol-promoted stimulation of NO release in cultured preoptic neurons. Thus, estrogen receptor-mediated stimulation of the nNOS/PSD-95/NMDA receptor complex assembly is likely to be a critical component of the signaling process by which estradiol facilitates coupling of glutamatergic fluxes for NO production in neurons.
Figures
Similar articles
-
Phosphorylation of N-methyl-D-aspartic acid receptor-associated neuronal nitric oxide synthase depends on estrogens and modulates hypothalamic nitric oxide production during the ovarian cycle.Endocrinology. 2010 Jun;151(6):2723-35. doi: 10.1210/en.2010-0007. Epub 2010 Apr 6. Endocrinology. 2010. PMID: 20371700 Free PMC article.
-
Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction.J Neurosci. 2007 Jun 6;27(23):6103-14. doi: 10.1523/JNEUROSCI.5595-06.2007. J Neurosci. 2007. PMID: 17553983 Free PMC article.
-
Enhancement of nitric oxide production by association of nitric oxide synthase with N-methyl-D-aspartate receptors via postsynaptic density 95 in genetically engineered Chinese hamster ovary cells: real-time fluorescence imaging using nitric oxide sensitive dye.J Neurochem. 2006 Mar;96(6):1531-9. doi: 10.1111/j.1471-4159.2006.03656.x. Epub 2006 Feb 8. J Neurochem. 2006. PMID: 16464237
-
Targeting neuronal nitric oxide synthase and the nitrergic system in post-traumatic stress disorder.Psychopharmacology (Berl). 2022 Oct;239(10):3057-3082. doi: 10.1007/s00213-022-06212-7. Epub 2022 Aug 27. Psychopharmacology (Berl). 2022. PMID: 36029333 Review.
-
Nitric Oxide Pathways in Neurovascular Coupling Under Normal and Stress Conditions in the Brain: Strategies to Rescue Aberrant Coupling and Improve Cerebral Blood Flow.Front Physiol. 2021 Oct 22;12:729201. doi: 10.3389/fphys.2021.729201. eCollection 2021. Front Physiol. 2021. PMID: 34744769 Free PMC article. Review.
Cited by
-
Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus.J Comp Neurol. 2017 Oct 15;525(15):3177-3189. doi: 10.1002/cne.24257. Epub 2017 Jun 19. J Comp Neurol. 2017. PMID: 28577305 Free PMC article.
-
Neuronal nitric oxide synthase and affective disorders.IBRO Rep. 2018 Nov 17;5:116-132. doi: 10.1016/j.ibror.2018.11.004. eCollection 2018 Dec. IBRO Rep. 2018. PMID: 30591953 Free PMC article. Review.
-
Supportive care of female hormones in brain health: what and how?Front Pharmacol. 2024 Jul 24;15:1403969. doi: 10.3389/fphar.2024.1403969. eCollection 2024. Front Pharmacol. 2024. PMID: 39114348 Free PMC article. Review.
-
Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation.J Neurosci. 2012 Jan 18;32(3):932-45. doi: 10.1523/JNEUROSCI.4765-11.2012. J Neurosci. 2012. PMID: 22262891 Free PMC article.
-
Nitric Oxide Plays a Key Role in Ovariectomy-Induced Apoptosis in Anterior Pituitary: Interplay between Nitric Oxide Pathway and Estrogen.PLoS One. 2016 Sep 9;11(9):e0162455. doi: 10.1371/journal.pone.0162455. eCollection 2016. PLoS One. 2016. PMID: 27611913 Free PMC article.
References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. - PubMed
-
- Abu-Soud HM, Yoho LL, Stuehr DJ. Calmodulin controls neuronal nitric-oxide synthase by a dual mechanism. Activation of intra- and interdomain electron transfer. J Biol Chem. 1994;269:32047–32050. - PubMed

