Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus
- PMID: 19187465
- PMCID: PMC2796511
- DOI: 10.1111/j.1365-2826.2009.01846.x
Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus
Abstract
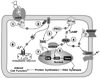
It is increasingly evident that 17beta-oestradiol (E(2)), via a distinct membrane oestrogen receptor (Gq-mER), can rapidly activate kinase pathways to have multiple downstream actions in central nervous system (CNS) neurones. We have found that E(2) can rapidly reduce the potency of the GABA(B) receptor agonist baclofen and mu-opioid receptor agonist DAMGO to activate G-protein-coupled, inwardly rectifying K(+) (GIRK) channels in hypothalamic neurones, thereby increasing the excitability (firing activity) of pro-opiomelanocortin (POMC) and dopamine neurones. These effects are mimicked by the membrane impermeant E(2)-BSA and a new ligand (STX) that is selective for the Gq-mER that does not bind to ERalpha or ERbeta. Both E(2) and STX are fully efficacious in attenuating the GABA(B) response in ERalpha, ERbeta and GPR 30 knockout mice in an ICI 182 780 reversible manner. These findings are further proof that E(2) signals through a unique plasma membrane ER. We have characterised the coupling of this Gq-mER to a Gq-mediated activation of phospholipase C leading to the up-regulation of protein kinase Cdelta and protein kinase A activity in these neurones, which ultimately alters gene transcription. Finally, as proof of principle, we have found that STX, similar to E(2), reduces food intake and body weight gain in ovariectomised females. STX, presumably via the Gq-mER, also regulates gene expression of a number of relevant targets including cation channels and signalling molecules that are critical for regulating (as a prime example) POMC neuronal excitability. Therefore, E(2) can activate multiple receptor-mediated pathways to modulate excitability and gene transcription in CNS neurones that are critical for controlling homeostasis and motivated behaviors.
Figures
References
-
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. - PubMed
-
- Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. - PubMed
-
- Hammes SR, Levin ER. Extra-nuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. - PubMed
-
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. - PubMed
-
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

