Intravenous tolerance modulates macrophage classical activation and antigen presentation in experimental autoimmune encephalomyelitis
- PMID: 19187972
- PMCID: PMC2723950
- DOI: 10.1016/j.jneuroim.2009.01.002
Intravenous tolerance modulates macrophage classical activation and antigen presentation in experimental autoimmune encephalomyelitis
Abstract
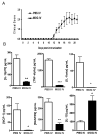
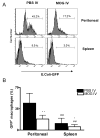
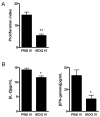
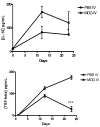
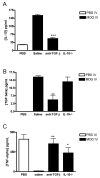
Macrophages act as the first line of self defense by mounting an inflammatory response to antigen and as antigen presenting cells to initiate the adaptive immune response. Inhibition of macrophage activation is one of the possible approaches to modulate inflammation. Intravenous (i.v.) tolerance has proved to be an effective method for ameliorating experimental autoimmune diseases. Whether macrophages are involved in tolerance induction is still largely undefined. In the present study we found that i.v. tolerance induction resulted in lower B7.1, B7.2 and MHC class II molecules, and reduced phagocytosis by both peritoneal macrophages and adherent splenocytes. Macrophages from tolerized mice were associated with a significantly impaired response of MOG-sensitized T cells to MOG. Macrophages from tolerized mice produced low levels of pro-inflammatory molecules IL-12, TNF-alpha, IL-1beta, RANTES and MCP-1 and high levels of IL-10 and TGF-beta. Administration of anti-TGF-beta led to a reduction of IL-10 in tolerized mice. Thus, i.v. tolerance inhibits macrophage classical activation and APC function, increases macrophage alternative activation and IL-10 and TGF-beta production. These cytokines, in turn, induce enhanced production of IL-10 in macrophages in MOG i.v. mice.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Allam R, Anders HJ. The role of innate immunity in autoimmune tissue injury. Curr Opin Rheumatol. 2008;20:538–544. - PubMed
-
- Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002;8:477–481. - PubMed
-
- Carlsen HS, Yamanaka T, Scott H, Rugtveit J, Brandtzaeg P. The proportion of CD40+ mucosal macrophages is increased in inflammatory bowel disease whereas CD40 ligand (CD154)+ T cells are relatively decreased, suggesting differential modulation of these costimulatory molecules in human gut lamina propria. Inflamm Bowel Dis. 2006;12:1013–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

