CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies
- PMID: 19188153
- PMCID: PMC2742326
- DOI: 10.1158/1078-0432.CCR-08-1456
CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies
Abstract
Purpose: To compare the in vitro and in vivo efficacy of CAT-8015, a second-generation recombinant immunotoxin composed of disulfide-linked affinity matured V(H) and V(L) chains of the mouse anti-CD22 monoclonal antibody RFB4 fused to PE38, to the parental compound CAT-3888.
Experimental design: The biological activity of CAT-8015 was examined in vitro using B-cell tumor lines and in vivo in a JD38-based s.c. tumor model in NCr athymic mice. Pharmacokinetics and interspecies scaling of CAT-8015 were evaluated in mice, rats, and cynomolgus monkeys. The potential toxicity of CAT-8015 was assessed in monkeys in a toxicologic study and compared with CAT-3888.
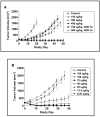
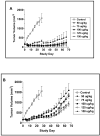
Results: The IC50 values of CAT-8015 in vitro using the EHEB, MEC1, Daudi, CA46, and JD38 cell lines ranged from 0.3 to 8.6 ng/mL. Pharmacokinetic studies with CAT-8015 were conducted in mouse, rat, and cynomolgus monkey. The t1/2 was calculated to be 0.42, 0.61, and 0.79 hours and the Vss was 1.37, 5.57, and 140.3 mL in mouse, rat, and monkey, respectively. In vivo, when JD38 tumor-bearing animals were treated with CAT-8015 at doses > or =75 microg/kg at 48-hour intervals for a total of three doses, a rapid reduction in tumor volume and in some cases complete remission in tumor growth was observed. The comparative toxicologic study showed comparable clinical and anatomic pathology changes for CAT-8015 and CAT-3888.
Conclusions: CAT-8015 is a CD22-targeting immunotoxin that, in preclinical studies, has greatly improved efficacy compared with CAT-3888.
Figures




References
-
- Carter P. Improving the efficacy of antibody-based cancer therapies. Nature Rev Cancer. 2001;1:118–129. - PubMed
-
- Schiavo G, Gisou van der Goot F. The bacterial toxin tool kit. Nature Rev. 2001;2:530–537. - PubMed
-
- Law CL, Cerveny CG, Gordon KA, et al. Efficient elimination of B-lineage lymphomas by anti-CD20-auristatin conjugates. Clin Cancer Res. 2004;10:7842–7851. - PubMed
-
- Senter PD, Springer CJ. Selective activation of anticancer prodrugs by monoclonal antibody-enzyme conjugates. Adv Drug Delivery Reviews. 2001;53:247–264. - PubMed
-
- Pastan I, Hassan R, FitzGerald DJ, et al. Immunotoxin therapy of Cancer. Nature Rev Cancer. 2006;6:559–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

