MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma
- PMID: 19188164
- PMCID: PMC7322626
- DOI: 10.1158/1078-0432.CCR-08-0399
MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma
Abstract
Purpose: Mantle cell lymphoma (MCL) has one of the poorest prognoses of the non-Hodgkin's lymphomas, and novel therapeutic approaches are needed. We wished to determine whether Nutlin-3, a novel small-molecule murine double minute 2 (MDM2) antagonist that efficiently activates TP53, might be effective in inducing cell death in MCL.
Experimental design: MCL cell lines with known TP53 status were treated with Nutlin-3, and biological and biochemical consequences were studied. Synergies with the prototypic genotoxic agent doxorubicin and the novel proteasome inhibitor bortezomib were assessed.
Results: Nutlin-3 resulted in a reduction in cell proliferation/viability (IC50 < 10 micromol/L), an increase in the apoptotic fraction, and cell cycle arrest in wild-type (wt) TP53 Z-138 and Granta 519 cells. These effects were accompanied by TP53 accumulation and induction of TP53-dependent proteins p21, MDM2, Puma, and Noxa. Cell cycle arrest was characterized by suppression of S phase and an increase in the G0-G1 and G2-M fractions and accompanied by suppression of total and phosphorylated retinoblastoma protein and a decrease in G2-M-associated proteins cyclin B and CDC2. The combination of Nutlin-3 with doxorubicin or bortezomib was synergistic in wt-TP53 MCL cells. Nutlin-3 also induced cell cycle arrest and reduced cell viability in the mutant TP53 MINO cells but at a significantly higher IC50 (22.5 micromol/L). These effects were associated with induction of the TP53 homologue p73, slight increases in p21 and Noxa, and caspase activation. Nutlin-3 and bortezomib synergistically inhibited cell growth of MINO.
Conclusion: These findings suggest that the MDM2 antagonist Nutlin-3 may be an effective agent in the treatment of MCL with or without wt-TP53.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures
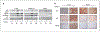


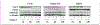


References
-
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer 2002;2:594–604. - PubMed
-
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 2006;6:909–23. - PubMed
-
- Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004;303:844–8. - PubMed
-
- Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood 2006;108:993–1000. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

