Effect of intracellular lipid droplets on cytosolic Ca2+ and cell death during ischaemia-reperfusion injury in cardiomyocytes
- PMID: 19188253
- PMCID: PMC2675001
- DOI: 10.1113/jphysiol.2008.163311
Effect of intracellular lipid droplets on cytosolic Ca2+ and cell death during ischaemia-reperfusion injury in cardiomyocytes
Abstract
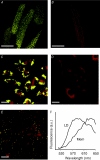
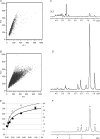
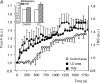
Lipid droplets (LD) consist of accumulations of triacylglycerols and have been proposed to be markers of ischaemic but viable tissue. Previous studies have described the presence of LD in myocardium surviving an acute coronary occlusion. We investigated whether LD may be protective against cell death secondary to ischaemia-reperfusion injury. The addition of oleate-bovine serum albumin complex to freshly isolated adult rat cardiomyocytes or to HL-1 cells resulted in the accumulation of intracellular LD detectable by fluorescence microscopy, flow cytometry and (1)H-nuclear magnetic resonance spectroscopy. Simulated ischaemia-reperfusion of HL-1 cells (respiratory inhibition at pH 6.4 followed by 30 min of reperfusion) resulted in significant cell death (29.7+/-2.6% of total lactate dehydrogenase release). However, cell death was significantly attenuated in cells containing LD (40% reduction in LDH release compared with control cells, P=0.02). The magnitude of LD accumulation was inversely correlated (r(2)=0.68, P=0.0003) with cell death. The protection associated with intracellular LD was not a direct effect of the fatty acids used to induce their formation, because oleate added 30 min before ischaemia, during ischaemia or during reperfusion did not form LD and did not protect against cell death. Increasing the concentration of free oleate during reperfusion progressively decreased the protection afforded by LD. HL-1 cells labelled with fluo-4, a Ca(2+)-sensitive fluorochrome, fluorescence within LD areas increased more throughout simulated ischaemia and reperfusion than in the cytosolic LD-free areas of the same cells. As a consequence, cells with LD showed less cytosolic Ca(2+) overload than control cells. These results suggest that LD exert a protective effect during ischaemia-reperfusion by sequestering free fatty acids and Ca(2+).
Figures



References
-
- Awonusonu F, Srinivasan S, Strange J, Al-Jumaily W, Bruce MC. Developmental shift in the relative percentages of fibroblast subsets: role of apoptosis postseptation. Am J Physiol Lung Cell Mol Physiol. 1999;277:L848–L859. - PubMed
-
- Barba I, Cabanas ME, Arus C. The relationship between nuclear magnetic resonance-visible lipids, lipid droplets, and cell proliferation in cultured C6 cells. Cancer Res. 1999;59:1861–1868. - PubMed
-
- Barrabes JA, Garcia-Dorado D, Ruiz-Meana M, Piper HM, Solares J, Gonzalez MA, Oliveras J, Herrejon MP, Soler J. Myocardial segment shrinkage during coronary reperfusion in situ. Relation to hypercontracture and myocardial necrosis. Pflugers Arch. 1996;431:519–526. - PubMed
-
- Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta. 2004;1742:119–131. - PubMed
-
- Bush KT, Lee H, Nagele RG. Lipid droplets of neuroepithelial cells are a major calcium storage site during neural tube formation in chick and mouse embryos. Experientia. 1992;48:516–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

