Increased sensitivity to iron deficiency in Arabidopsis thaliana overaccumulating nicotianamine
- PMID: 19188276
- PMCID: PMC2657549
- DOI: 10.1093/jxb/erp007
Increased sensitivity to iron deficiency in Arabidopsis thaliana overaccumulating nicotianamine
Abstract
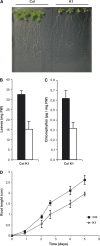
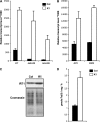
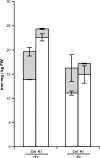
Nicotianamine (NA) is a non-protein amino acid derivative synthesized from S-adenosyl L-methionine able to bind several metal ions such as iron, copper, manganese, zinc, or nickel. In plants, NA appears to be involved in iron availability and is essential for the plant to complete its biological cycle. In graminaceous plants, NA is also the precursor in the biosynthesis of phytosiderophores. Arabidopsis lines accumulating 4- and 100-fold more NA than wild-type plants were used in order to evaluate the impact of such an NA overaccumulation on iron homeostasis. The expression of iron-regulated genes including the IRT1/FRO2 iron uptake system is highly induced at the transcript level under both iron-sufficient and iron-deficient conditions. Nevertheless, NA overaccumulation does not interfere with the iron uptake mechanisms since the iron levels are similar in the NA-overaccumulating line and wild-type plants in both roots and leaves under both sufficient and deficient conditions. This observation also suggests that the translocation of iron from the root to the shoot is not affected in the NA-overaccumulating line. However, NA overaccumulation triggers an enhanced sensitivity to iron starvation, associated with a decrease in iron availability. This study draws attention to a particular phenotype where NA in excess paradoxically leads to iron deficiency, probably because of an increase of the NA apoplastic pool sequestering iron. This finding strengthens the notion that extracellular NA in the apoplast could be a major checkpoint to control plant iron homeostasis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

