Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice
- PMID: 19188353
- PMCID: PMC2663167
- DOI: 10.1128/IAI.00814-08
Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice
Abstract
Enterotoxigenic Bacteroides fragilis (ETBF) causes diarrhea and is implicated in inflammatory bowel diseases and colorectal cancer. The only known ETBF virulence factor is the Bacteroides fragilis toxin (BFT), which induces E-cadherin cleavage, interleukin-8 secretion, and epithelial cell proliferation. A murine model for ETBF has not been characterized. Specific pathogen-free (SPF) C57BL/6J or germfree 129S6/SvEv mice were orally inoculated with wild-type ETBF (WT-ETBF) strains, a nontoxigenic WT strain of B. fragilis (WT-NTBF), WT-NTBF overexpressing bft (rETBF), or WT-NTBF overexpressing a biologically inactive mutated bft (rNTBF). In SPF and germfree mice, ETBF caused colitis but was lethal only in germfree mice. Colonic histopathology demonstrated mucosal thickening with inflammatory cell infiltration, crypt abscesses, and epithelial cell exfoliation, erosion, and ulceration. SPF mice colonized with rETBF mimicked WT-ETBF, whereas rNTBF caused no histopathology. Intestinal epithelial E-cadherin was rapidly cleaved in vivo in WT-ETBF-colonized mice and in vitro in intestinal tissues cultured with purified BFT. ETBF mice colonized for 16 months exhibited persistent colitis. BFT did not directly induce lymphocyte proliferation, dendritic cell stimulation, or Toll-like receptor activation. In conclusion, WT-ETBF induced acute then persistent colitis in SPF mice and rapidly lethal colitis in WT germfree mice. Our data support the hypothesis that chronic colonization with the human commensal ETBF can induce persistent, subclinical colitis in humans.
Figures




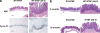

References
-
- Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 712839-2858. - PMC - PubMed
-
- Basset, C., J. Holton, A. Bazeos, D. Vaira, and S. Bloom. 2004. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig. Dis. Sci. 491425-1432. - PubMed
-
- Coyne, M. J., W. Kalka-Moll, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2000. Bacteroides fragilis NCTC9343 produces at least three distinct capsular polysaccharides: cloning, characterization, and reassignment of polysaccharide B and C biosynthesis loci. Infect. Immun. 686176-6181. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR020764/RR/NCRR NIH HHS/United States
- HHSN261200433002C/PHS HHS/United States
- P50 CA062924/CA/NCI NIH HHS/United States
- R01 DK045496/DK/NIDDK NIH HHS/United States
- RR00171/RR/NCRR NIH HHS/United States
- RR21362/RR/NCRR NIH HHS/United States
- R01 DK45496/DK/NIDDK NIH HHS/United States
- K26 RR000171/RR/NCRR NIH HHS/United States
- P30 DK34987/DK/NIDDK NIH HHS/United States
- CA62924/CA/NCI NIH HHS/United States
- K01 A1066994/PHS HHS/United States
- R24 DK064388/DK/NIDDK NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- R01 DK053347/DK/NIDDK NIH HHS/United States
- K01 RR021362/RR/NCRR NIH HHS/United States
- P40 RR020764/RR/NCRR NIH HHS/United States
- R01 DK53347/DK/NIDDK NIH HHS/United States
- R01 DK080817/DK/NIDDK NIH HHS/United States
- R24 DK64388/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

