Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia
- PMID: 19188394
- PMCID: PMC2663089
- DOI: 10.1128/AAC.01141-08
Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia
Abstract
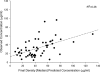
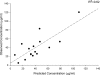
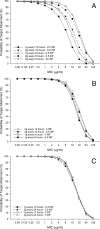
A population pharmacokinetic model of cefepime was constructed from data from adult critical care patients with ventilator-associated pneumonia (VAP). A total of 32 patients treated with high-dose cefepime, 2 g every 8 h (3-h infusion) or a renal function-adjusted equivalent dose, were randomized into two groups--26 for the initial model and 6 for model validation. Serum samples of cefepime were collected at steady state. Nonparametric adaptive grid population modeling was employed using a two-compartment K(slope) pharmacokinetic model relating the elimination rate constant (K(10)) to renal function, as defined by creatinine clearance (CL(CR)), and central distribution volume (V(1)) to total body weight (TBW). The final model was described by the following equations: K(10) = 0.0027 x CL(CR) + 0.071 h(-1) and V(1) = TBW x 0.21 liter/kg. The median intercompartmental transfer constants K(12) and K(21) were 0.780 h(-1) and 0.472 h(-1), respectively. Using these median parameter estimates, the bias, precision, and coefficient of determination for the initial model were 11.3 microg/ml, 24.0 microg/ml, and 26%, respectively. The independent validation group displayed a bias, precision, and coefficient of determination of -1.64 microg/ml, 17.1 microg/ml, and 62%, respectively. Time-concentration profiles were assessed for various dosing regimens, using 5,000-patient Monte Carlo simulations. Among the regimens, the likelihoods of 2 g every 8 h (3-h infusion) achieving free drug concentrations above the MIC for 50% of the dosing interval were 91.8%, 78.1%, and 50.3% for MICs of 8, 16, and 32 microg/ml, respectively. This study provides a pharmacokinetic model capable of predicting cefepime concentrations in critically ill patients with VAP.
Figures
References
-
- Barradell, L. B., and H. M. Bryson. 1994. Cefepime: a review of its antimicrobial activity, pharmacokinetic properties and therapeutic use. Drugs 47:471-505. - PubMed
-
- Boselli, E., D. Breilh, F. Duflo, M. C. Saux, R. Debon, D. Chassard, and B. Allaouchiche. 2003. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit. Care Med. 31:2102-2106. - PubMed
-
- Burgess, D. S., R. W. Hastings, and T. C. Hardin. 2000. Pharmacokinetics and pharmacodynamics of cefepime administration by intermittent and continuous infusion. Clin. Ther. 22:66-75. - PubMed
-
- Bustad, A., D. Terziivanov, R. Leary, R. Port, A. Schumitzky, and R. Jelliffe. 2006. Parametric and nonparametric populations methods. Their comparative performance in analyzing a clinical dataset and two Monte Carlo simulation studies. Clin. Pharmacokinet. 45:365-383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

