Myocilin is a modulator of Wnt signaling
- PMID: 19188438
- PMCID: PMC2663295
- DOI: 10.1128/MCB.01274-08
Myocilin is a modulator of Wnt signaling
Abstract
It is well documented that mutations in the MYOCILIN gene may lead to juvenile- and adult-onset primary open-angle glaucoma. However, the functions of wild-type myocilin are still not well understood. To study the functions of human myocilin and its two proteolytic fragments, these proteins were expressed in HEK293 cells. Conditioned medium from myocilin-expressing cells, as well as purified myocilin, induced the formation of stress fibers in primary cultures of human trabecular meshwork or NIH 3T3 cells. Stress fiber-inducing activity of myocilin was blocked by antibodies against myocilin, as well as secreted inhibitors of Wnt signaling, secreted Frizzled-related protein 1 (sFRP1) or sFRP3, and beta-catenin small interfering RNA. Interaction of myocilin with sFRP1, sFRP3, and several Frizzled receptors was confirmed by immunoprecipitation experiments and by binding of myocilin to the surface of cells expressing cysteine-rich domains of different Frizzled and sFRPs. Treatment of NIH 3T3 cells with myocilin and its fragments induced intracellular redistribution of beta-catenin and its accumulation on the cellular membrane but did not induce nuclear accumulation of beta-catenin. Overexpression of myocilin in the eye angle tissues of transgenic mice stimulated accumulation of beta-catenin in these tissues. Myocilin and Wnt proteins may perform redundant functions in the mammalian eye, since myocilin modulates Wnt signaling by interacting with components of this signaling pathway.
Figures

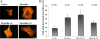










References
-
- Adam, M. F., A. Belmouden, P. Binisti, A. P. Brezin, F. Valtot, A. Bevhetoille, J.-C. Dascotte, B. Copin, L. Gomez, A. Chaventre, J.-F. Bach, and H.-J. Garchon. 1997. Recurrent mutations in a single exon encoding the evolutionary conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum. Mol. Genet. 62091-2097. - PubMed
-
- Alward, W. L., Y. H. Kwon, C. L. Khanna, A. T. Johnson, S. S. Hayreh, M. B. Zimmerman, J. Narkiewicz, J. L. Andorf, P. A. Moore, J. H. Fingert, V. C. Sheffield, and E. M. Stone. 2002. Variations in the myocilin gene in patients with open-angle glaucoma. Arch. Ophthalmol. 1201189-1197. - PubMed
-
- Aroca-Aguilar, J. D., F. Sanchez-Sanchez, S. Ghosh, M. Coca-Prados, and J. Escribano. 2005. Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J. Biol. Chem. 28021043-21051. - PubMed
-
- Barembaum, M., T. A. Moreno, C. LaBonne, J. Sechrist, and M. Bronner-Fraser. 2000. Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nature Cell Biol. 2219-225. - PubMed
-
- Barker, N., and H. Clevers. 2006. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 5997-1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
