Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation
- PMID: 19188501
- PMCID: PMC2735196
- DOI: 10.1161/CIRCULATIONAHA.108.817379
Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation
Abstract
Background: Recent evidence indicates that membrane voltage and Ca2+ clocks jointly regulate sinoatrial node (SAN) automaticity. Here we test the hypothesis that sinus rate acceleration by beta-adrenergic stimulation involves synergistic interactions between these clock mechanisms.
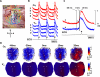
Methods and results: We simultaneously mapped intracellular calcium (Ca(i)) and membrane potential in 25 isolated canine right atrium, using previously described criteria of the timing of late diastolic Ca(i) elevation (LDCAE) relative to the action potential upstroke to detect the Ca2+ clock. Before isoproterenol, the earliest pacemaking site occurred in the inferior SAN, and LDCAE was observed in only 4 of 25 preparations. Isoproterenol infusion (1 micromol/L) increased sinus rate and shifted pacemaking site to superior SAN, concomitant with the appearance of LDCAE preceding the action potential upstroke by 98+/-31 ms. Caffeine had similar effects, whereas sarcoplasmic reticulum Ca2+ depletion with ryanodine and thapsigargin prevented isoproterenol-induced LDCAE and blunted sinus rate acceleration. Ca(i) transient relaxation time during isoproterenol was shorter in superior SAN (124+/-34 ms) than inferior SAN (138+/-24 ms; P=0.01) or right atrium (164+/-33 ms; P=0.001) and was associated with a lower sarcoplasmic reticulum Ca2+ ATPase pump to phospholamban protein ratio in SAN than in right atrium. Hyperpolarization-activated pacemaker current (I(f)) blockade with ZD 7288 modestly blunted but did not prevent LDCAE or sinus rate acceleration by isoproterenol.
Conclusions: Acceleration of the Ca2+ clock in the superior SAN plays an important role in sinus acceleration during beta-adrenergic stimulation, interacting synergistically with the voltage clock to increase sinus rate.
Figures







References
-
- Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–236. - PubMed
-
- Baruscotti M, Bucchi A, Difrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005;107:59–79. - PubMed
-
- Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circulation research. 2001;88:1254–1258. - PubMed
-
- Hata T, Noda T, Nishimura M, Watanabe Y. The role of Ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart Vessels. 1996;11:234–241. - PubMed
-
- Li J, Qu J, Nathan RD. Ionic basis of ryanodine's negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am J Physiol. 1997;273:H2481–2489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

