Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle
- PMID: 19188611
- PMCID: PMC2650171
- DOI: 10.1073/pnas.0811400106
Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle
Abstract
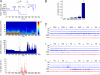
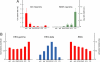
Neurons containing melanin-concentrating hormone (MCH) are codistributed with neurons containing orexin (Orx or hypocretin) in the lateral hypothalamus, a peptide and region known to be critical for maintaining wakefulness. Evidence from knockout and c-Fos studies suggests, however, that the MCH neurons might play a different role than Orx neurons in regulating activity and sleep-wake states. To examine this possibility, neurons were recorded across natural sleep-wake states in head-fixed rats and labeled by using the juxtacellular technique for subsequent immunohistochemical identification. Neurons identified as MCH+ did not fire during wake (W); they fired selectively during sleep, occasionally during slow wave sleep (SWS) and maximally during paradoxical sleep (PS). As W-Off/Sleep-On, the MCH neurons discharged in a reciprocal manner to the W-On/Sleep-Off Orx neurons and could accordingly play a complementary role to Orx neurons in sleep-wake state regulation and contribute to the pathophysiology of certain sleep disorders, such as narcolepsy with cataplexy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jones BE. In: Principles and Practice of Sleep Medicine. Kryger MH, Roth T, Dement WC, editors. Philadelphia: Elsevier Saunders; 2005. pp. 136–153.
-
- Chemelli RM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. - PubMed
-
- Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

