Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era
- PMID: 19188649
- PMCID: PMC2730663
- DOI: 10.1001/archpedi.163.2.164
Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era
Erratum in
- Arch Pediatr Adolesc Med. 2009 Apr;163(4):364
Abstract
Objective: To estimate highly active antiretroviral therapy (HAART)-era incident rates for the first episode of noninfectious conditions in human immunodeficiency virus (HIV)-infected youth in order to identify HAART-era changes in the natural history of perinatal HIV infection.
Design: Multicenter prospective cohort study.
Setting: More than 80 sites in the United States including Puerto Rico.
Patients: Perinatally HIV-infected youth.
Main outcome measures: Incidence rates (IRs) per 100 person-years were calculated for targeted noninfectious conditions occurring in perinatally HIV-infected children. A chi(2) test for linear trend was used to evaluate changes in the rates from 2001 to 2006.
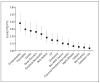
Results: Two thousand five hundred seventy-five perinatally HIV-infected children (51%, female; 59%, black, non-Hispanic) were enrolled in Pediatric AIDS Clinical Trials Group (PACTG) 219C between 2000 and 2006 and were followed up for a median of 59 months. The 10 most common noninfectious conditions were pregnancy conditions (IR = 6.16; 95% confidence interval (CI), 3.9-9.3), birth defects (IR = 0.19; 95% CI, 0.1-0.3), gynecological dysplasias (IR = 5.92; 95% CI, 3.9-8.6), condyloma (IR = 0.15; 95% CI, 0.1-0.2), encephalopathy (IR = 0.38; 95% CI, 0.3-0.5), pancreatitis (IR = 0.30; 95% CI, 0.2-0.4), cardiac disorders (IR = 0.28; 95% CI, 0.2-0.4), renal disorders (IR = 0.26; 95% CI, 0.2-0.4), peripheral neuropathy (IR = 0.23; 95% CI, 0.2-0.4), and idiopathic thrombocytic purpura (IR = 0.15; 95% CI, 0.1-0.3). Among these conditions, 5 showed significant trends, with IRs increasing over time in pregnancy-related conditions (P < .001) and gynecological dysplasias (P = .02) while IRs decreased over time for encephalopathy (P < .001), pancreatitis (P = .002), and cardiac disorders (P = .007).
Conclusions: Between 2001 and 2006, the incidence for 3 conditions decreased and increased for 2 others, demonstrating the change in medical issues and conditions in perinatally infected youth. Continued surveillance with appropriate tools will be needed to assess the long-term effects of HAART and HIV as well as development of new noninfectious conditions of HIV.
Figures

References
-
- Louie JK, Hsu LC, Osmond DH, Katz MH, Schwarcz SK. Trends in causes of death among persons with acquired immunodeficiency syndrome in the era of highly active antiretroviral therapy, San Francisco, 1994–1998. J Infect Dis. 2002;186(7):1023–1027. - PubMed
-
- Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. - PubMed
-
- Culnane M, Fowler MG, Lee S, et al. PACTG and Protocol 076/219 Teams. Lack of long term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. JAMA. 1999;281(2):151–157. - PubMed
-
- Gortmaker SL, Hughes M, Cervia J, et al. The impact of protease inhibitor combination therapy on mortality among children and youth infected with HIV-1. N Engl J Med. 2001;345(21):1522–1528. - PubMed
-
- Dankner WM, Lindsey JC, Levin MJ Pediatric AIDS Clinical Trials Group Protocol Teams 051, 128, 138, 144, 152, 179, 190, 220, 240, 245, 254, 300, and 327. Correlates of opportunistic infections in children infected with the human immunodeficiency virus managed before highly active antiretroviral therapy. Pediatr Infect Dis J. 2001;20(1):40–48. - PubMed

