Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress
- PMID: 19188685
- PMCID: PMC2648679
- DOI: 10.1172/JCI35977
Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress
Abstract
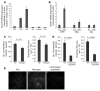
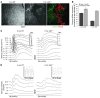
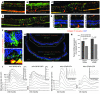
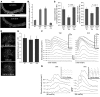
In several disease states, abnormal growth of blood vessels is associated with local neuronal degeneration. This is particularly true in ocular diseases such as retinal angiomatous proliferation (RAP) and macular telangiectasia (MacTel), in which, despite the absence of large-scale leakage or hemorrhage, abnormal neovascularization (NV) is associated with local neuronal dysfunction. We describe here a retinal phenotype in mice with dysfunctional receptors for VLDL (Vldlr-/- mice) that closely resembles human retinal diseases in which abnormal intra- and subretinal NV is associated with photoreceptor cell death. Such cell death was evidenced by decreased cone and, to a lesser extent, rod opsin expression and abnormal electroretinograms. Cell death in the region of intraretinal vascular abnormalities was associated with an increased presence of markers associated with oxidative stress. Oral antioxidant supplementation protected against photoreceptor degeneration and preserved retinal function, despite the continued presence of abnormal intra- and subretinal vessels. What we believe to be novel, Müller cell-based, virally mediated delivery of neurotrophic compounds specifically to sites of NV was also neuroprotective. These observations demonstrate that neuronal loss secondary to NV can be prevented by the use of simple antioxidant dietary measures or cell-based delivery of neurotrophic factors, even when the underlying vascular phenotype is not altered.
Figures



References
-
- Nunomura A., et al. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. - DOI - PubMed
-
- Faucheux B.A., Bonnet A.M., Agid Y., Hirsch E.C. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet. 1999;353:981–982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

