A reengineered hospital discharge program to decrease rehospitalization: a randomized trial
- PMID: 19189907
- PMCID: PMC2738592
- DOI: 10.7326/0003-4819-150-3-200902030-00007
A reengineered hospital discharge program to decrease rehospitalization: a randomized trial
Abstract
Background: Emergency department visits and rehospitalization are common after hospital discharge.
Objective: To test the effects of an intervention designed to minimize hospital utilization after discharge.
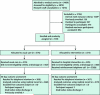
Design: Randomized trial using block randomization of 6 and 8. Randomly arranged index cards were placed in opaque envelopes labeled consecutively with study numbers, and participants were assigned a study group by revealing the index card.
Setting: General medical service at an urban, academic, safety-net hospital.
Patients: 749 English-speaking hospitalized adults (mean age, 49.9 years).
Intervention: A nurse discharge advocate worked with patients during their hospital stay to arrange follow-up appointments, confirm medication reconciliation, and conduct patient education with an individualized instruction booklet that was sent to their primary care provider. A clinical pharmacist called patients 2 to 4 days after discharge to reinforce the discharge plan and review medications. Participants and providers were not blinded to treatment assignment.
Measurements: Primary outcomes were emergency department visits and hospitalizations within 30 days of discharge. Secondary outcomes were self-reported preparedness for discharge and frequency of primary care providers' follow-up within 30 days of discharge. Research staff doing follow-up were blinded to study group assignment.
Results: Participants in the intervention group (n = 370) had a lower rate of hospital utilization than those receiving usual care (n = 368) (0.314 vs. 0.451 visit per person per month; incidence rate ratio, 0.695 [95% CI, 0.515 to 0.937]; P = 0.009). The intervention was most effective among participants with hospital utilization in the 6 months before index admission (P = 0.014). Adverse events were not assessed; these data were collected but are still being analyzed.
Limitation: This was a single-center study in which not all potentially eligible patients could be enrolled, and outcome assessment sometimes relied on participant report.
Conclusion: A package of discharge services reduced hospital utilization within 30 days of discharge.
Funding: Agency for Healthcare Research and Quality and National Heart, Lung, and Blood Institute, National Institutes of Health.
Trial registration: ClinicalTrials.gov NCT00252057.
Figures

Comment in
-
The science of safety improvement: learning while doing.Ann Intern Med. 2011 May 17;154(10):699-701. doi: 10.7326/0003-4819-154-10-201105170-00013. Ann Intern Med. 2011. PMID: 21576540 No abstract available.
References
-
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–7. [PMID: 12558354] - PubMed
-
- Greenwald JL, Denham CR, Jack BW. The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process. J Patient Saf. 2007;3:97–106.
-
- Wachter RM. Hospitalists in the United States—mission accomplished or work in progress? N Engl J Med. 2004;350:1935–6. [PMID: 15128892] - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
