Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program
- PMID: 19190248
- PMCID: PMC2668845
- DOI: 10.1182/blood-2008-08-175323
Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program
Abstract
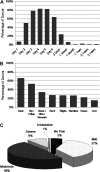
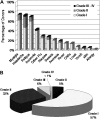
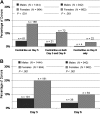
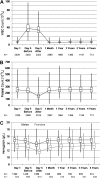
Limited data are available describing donor adverse events (AEs) associated with filgrastim mobilized peripheral blood stem cell (PBSC) collections in unrelated volunteers. We report results in 2408 unrelated PBSC donors prospectively evaluated by the National Marrow Donor Program (NMDP) between 1999 and 2004. Female donors had higher rates of AEs, requiring central line placement more often (17% vs 4%, P< .001), experiencing more apheresis-related AEs (20% vs 7%, P< .001), more bone pain (odds ratio [OR]=1.49), and higher rates of grades II-IV and III-IV CALGB AEs (OR=2.22 and 2.32). Obese donors experienced more bone pain (obese vs normal, OR=1.73) and heavy donors had higher rates of CALGB toxicities (>95 kg vs <70 kg, OR=1.49). Six percent of donors experienced grade III-IV CALGB toxicities and 0.6% experienced toxicities that were considered serious and unexpected. Complete recovery is universal, however, and no late AEs attributable to donation have been identified. In conclusion, PBSC collection in unrelated donors is generally safe, but nearly all donors will experience bone pain, 1 in 4 will have significant headache, nausea, or citrate toxicity, and a small percentage will experience serious short-term adverse events. In addition, women and larger donors are at higher risk for donation-related AEs.
Figures

References
-
- Craddock C, Szydlo RM, Klein JP, et al. Estimating leukemia-free survival after allografting for chronic myeloid leukemia: a new method that takes into account patients who relapse and are restored to complete remission. Blood. 2000;96:86–90. - PubMed
-
- Karlsson L, Quinlan D, Guo D, et al. Mobilized blood cells vs bone marrow harvest: experience compared in 171 donors with particular reference to pain and fatigue. Bone Marrow Transplant. 2004;33:709–713. - PubMed
-
- Cesaro S, Marson P, Gazzola MV, et al. The use of cytokine-stimulated healthy donors in allogeneic stem cell transplantation. Haematologica. 2002;87:35–41. - PubMed
-
- Anderlini P, Przepiorka D, Lauppe J, et al. Collection of peripheral blood stem cells from normal donors 60 years of age or older. Br J Haematol. 1997;97:485–487. - PubMed
-
- Pulsipher MA, Levine JE, Hayashi RJ, et al. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: the pediatric blood and marrow transplant consortium experience (PBMTC) 1996-2003. Bone Marrow Transplant. 2005;35:361–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

