Identification of a novel pathway that selectively modulates apoptosis of breast cancer cells
- PMID: 19190336
- PMCID: PMC4264605
- DOI: 10.1158/0008-5472.CAN-08-2896
Identification of a novel pathway that selectively modulates apoptosis of breast cancer cells
Abstract
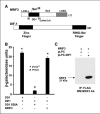
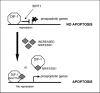
Expression of the nuclear receptor interacting factor 3 (NRIF3) coregulator in a wide variety of breast cancer cells selectively leads to rapid caspase-2-dependent apoptotic cell death. A novel death domain (DD1) was mapped to a 30-amino acid region of NRIF3. Because the cytotoxicity of NRIF3 and DD1 seems to be cell type-specific, these studies suggest that breast cancer cells contain a novel "death switch" that can be specifically modulated by NRIF3 or DD1. Using an MCF-7 cell cDNA library in a yeast two-hybrid screen, we cloned a factor that mediates apoptosis by DD1 and refer to this factor as DD1-interacting factor-1 (DIF-1). DIF-1 is a transcriptional repressor that mediates its effect through SirT1, and this repression is attenuated by the binding of NRIF3/DD1. DIF-1 expression rescues breast cancer cells from NRIF3/DD1-induced apoptosis. Small interfering RNA (siRNA) knockdown of DIF-1 selectively leads to apoptosis of breast cancer cells, further suggesting that DIF-1 plays a key role in NRIF3/DD1-mediated apoptosis. A protein kinase A inhibitor (H89) also elicits apoptosis of breast cancer cells but not of the other cell types examined, and DIF-1 also protects these cells from H89-mediated apoptosis. In addition, H89 incubation results in a rapid increase in NRIF3 levels and siRNA knockdown of NRIF3 protects breast cancer cells from H89-mediated apoptosis. Our results indicate that DIF-1 plays a key role in breast cancer cell survival and further characterizing this pathway may provide important insights into developing novel therapies to selectively target breast cancer cells for apoptosis.
Figures

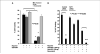


References
-
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. - PubMed
-
- Lahm A, Paradisi A, Green DR, Melino G. Death fold domain interaction in apoptosis. Cell Death Differ. 2003;10:10–2. - PubMed
-
- Afonja A, Raaka BM, Huang A, et al. RAR agonists stimulate SOX9 gene expression in breast cancer cell lines: evidence for a role in retinoid-mediated growth inhibition. Oncogene. 2002;21:7850–60. - PubMed
-
- Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene. 2004;23:8135–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

