Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen
- PMID: 19190349
- PMCID: PMC2644349
- DOI: 10.1158/0008-5472.CAN-08-0857
Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen
Abstract
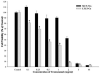
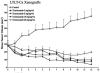
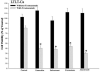
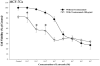
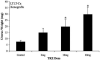
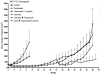
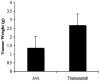
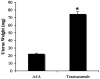
In this study, we investigated adaptive mechanisms associated with aromatase inhibitor (AI) resistance in breast cancer cells and show that sensitivity to AIs can be extended through dual inhibition of estrogen receptor (ER) and human epidermal receptor-2 (Her-2) signaling. We used human ER-positive breast cancer cells stably transfected with the aromatase gene (MCF-7Ca). These cells grow as tumors in nude mice and are inhibited by AIs. Despite continued treatment, tumors eventually become insensitive to AI letrozole. The cells isolated from these long-term letrozole-treated tumors (LTLT-Ca) were found to have decreased ERalpha levels. Our results suggest that LTLT-Ca cells survive estrogen deprivation by activation of Her-2/mitogen-activated protein kinase (MAPK) pathway. Here, we show that trastuzumab (antibody against Her-2; IC(50) = 0.4 mg/mL) was very effective in restoring the ERalpha levels and sensitivity of LTLT-Ca cells to endocrine therapy by down-regulation of Her-2/MAPK pathway and up-regulation of ERalpha. In contrast, trastuzumab was ineffective in the parental hormone-responsive MCF-7Ca cells (IC(50) = 4.28 mg/mL) and xenografts. By blocking Her-2, trastuzumab also up-regulates ERalpha and aromatase expression and hypersensitized MCF-7Ca cells to E(2). We show that trastuzumab is beneficial in hormone-refractory cells and xenografts by restoring ER, implicating Her-2 as a negative regulator of ERalpha. In xenograft studies, the combination of trastuzumab plus letrozole is equally effective in inhibiting growth of MCF-7Ca tumors as letrozole alone. However, on the acquisition of resistance and increased Her-2 expression, the combination of letrozole plus trastuzumab provided superior benefit over letrozole or trastuzumab alone.
Figures


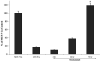




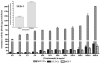

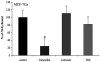
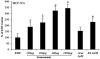




References
-
- Jelovac D, Sabnis G, Long BJ, et al. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005;65:5380–9. - PubMed
-
- Long BJ, Jelovac D, Handratta V, et al. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J Natl Cancer Inst. 2004;96:456–65. - PubMed
-
- Long BJ, Jelovac D, Thiantanawat A, Brodie AM. The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with the aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clin Cancer Res. 2002;8:2378–88. - PubMed
-
- Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–10. - PubMed
-
- Shim WS, Conaway M, Masamura S, et al. Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology. 2000;141:396–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

