Computational studies of the O(2)-evolving complex of photosystem II and biomimetic oxomanganese complexes
- PMID: 19190716
- PMCID: PMC2350217
- DOI: 10.1016/j.ccr.2007.09.006
Computational studies of the O(2)-evolving complex of photosystem II and biomimetic oxomanganese complexes
Abstract
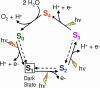
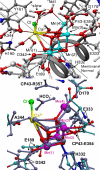
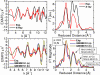
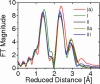
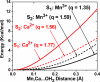
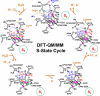
In recent years, there has been considerable interest in studies of catalytic metal clusters in metalloproteins based on Density Functional Theory (DFT) quantum mechanics/molecular mechanics (QM/MM) hybrid methods. These methods explicitly include the perturbational influence of the surrounding protein environment on the structural/functional properties of the catalytic centers. In conjunction with recent breakthroughs in X-ray crystallography and advances in spectroscopic and biophysical studies, computational chemists are trying to understand the structural and mechanistic properties of the oxygen-evolving complex (OEC) embedded in photosystem II (PSII). Recent studies include the development of DFT-QM/MM computational models of the Mn(4)Ca cluster, responsible for photosynthetic water oxidation, and comparative quantum mechanical studies of biomimetic oxomanganese complexes. A number of computational models, varying in oxidation and protonation states and ligation of the catalytic center by amino acid residues, water, hydroxide and chloride have been characterized along the PSII catalytic cycle of water splitting. The resulting QM/MM models are consistent with available mechanistic data, Fourier-transform infrared (FTIR) spectroscopy, X-ray diffraction data and extended X-ray absorption fine structure (EXAFS) measurements. Here, we review these computational efforts focused towards understanding the catalytic mechanism of water oxidation at the detailed molecular level.
Figures






References
-
- Joliot P, Barbieri G, Chabaud R. Photochem. Photobiol. 1969;10:309.
-
- Kok B, Forbush B, McGloin M. Photochem. Photobiol. 1970;11:457. - PubMed
-
- Diner BA, Babcock GT. Structure, Dynamics, and Energy Conversion Efficiency in Photosystem II. In: Ort DR, Yocum CF, editors. Oxygenic Photosynthesis: The Light Reactions. Kluwer Academic Publishers; Dordrecht: 1996. p. 213.
-
- Barber J. Quart. Rev. Biophys. 2003;36:71. - PubMed
-
- Yachandra VK, Sauer K, Klein MP. Chem. Rev. 1996;96:2927. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
