anti-Diastereo- and enantioselective carbonyl crotylation from the alcohol or aldehyde oxidation level employing a cyclometallated iridium catalyst: alpha-methyl allyl acetate as a surrogate to preformed crotylmetal reagents
- PMID: 19191498
- PMCID: PMC3165013
- DOI: 10.1021/ja808857w
anti-Diastereo- and enantioselective carbonyl crotylation from the alcohol or aldehyde oxidation level employing a cyclometallated iridium catalyst: alpha-methyl allyl acetate as a surrogate to preformed crotylmetal reagents
Abstract
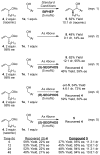
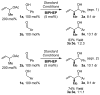
Under the conditions of transfer hydrogenation employing an ortho-cyclometallated iridium catalyst generated in situ from [Ir(cod)Cl](2), 4-cyano-3-nitrobenzoic acid and the chiral phosphine ligand (S)-SEGPHOS, alpha-methyl allyl acetate couples to alcohols 1a-1j with complete levels of branched regioselectivity to furnish products of carbonyl crotylation 3a-3j, which are formed with good levels of anti-diastereoselectivity and exceptional levels of enantioselectivity. An identical set of optically enriched carbonyl crotylation products 3a-3j is accessible from the corresponding aldehydes 2a-2j under the same conditions, but employing isopropanol as the terminal reductant. Experiments aimed at probing the origins of stereoselection establish a matched mode of ionization for the (R)-acetate and the iridium catalyst modified by (S)-SEGPHOS, as well as reversible ionization of the allylic acetate with rapid pi-facial interconversion of the resulting pi-crotyl intermediate in advance of C-C bond formation. Additionally, rapid alcohol-aldehyde redox equilibration in advance of carbonyl addition is demonstrated. Thus, anti-diastereo- and enantioselective carbonyl crotylation from the alcohol or aldehyde oxidation level is achieved in the absence of any stoichiometric metallic reagents or stoichiometric metallic byproducts.
Figures

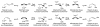
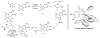
References
-
-
For selected reviews on enantioselective carbonyl allylation and crotylation, see: Hoffmann RW. Angew Chem, Int Ed Engl. 1982;21:555.Yamamoto Y, Asao N. Chem Rev. 1993;93:2207.Ramachandran PV. Aldrichim Acta. 2002;35:23.Kennedy JWJ, Hall DG. Angew Chem, Int Ed. 2003;42:4732.Denmark SE, Fu J. Chem Rev. 2003;103:2763.Yu CM, Youn J, Jung HK. Bull Korean Chem Soc. 2006;27:463.Marek I, Sklute G. Chem Commun. 2007:1683.Hall DG. Synlett. 2007:1644.
-
-
-
For enantioselective carbonyl additions of chirally modified crotylmetal reagents, see: Hoffmann RW, Ladner W. Tetrahedron Lett. 1979;20:4653.Brown HC, Bhat KS. J Am Chem Soc. 1986;108:293.Brown HC, Bhat KS. J Am Chem Soc. 1986;108:5919.Roush WR, Halterman RL. J Am Chem Soc. 1986;108:294.Roush WR, Ando K, Powers DB, Palkowitz AD, Halterman RL. J Am Chem Soc. 1990;112:6339.Garcia J, Kim BM, Masamune S. J Org Chem. 1987;52:4831.Burgos CH, Canales E, Matos K, Soderquist JA. J Am Chem Soc. 2005;127:8044.Riediker M, Duthaler RO. Angew Chem, Int Ed Engl. 1989;28:494.Hafner A, Duthaler RO, Marti R, Rihs G, Rothe-Streit P, Schwarzenbach F. J Am Chem Soc. 1992;114:2321.Panek JS, Yang M. J Am Chem Soc. 1991;113:6594.Hu T, Takenaka N, Panek JS. J Am Chem Soc. 2002;124:12806.Hackman BM, Lombardi PJ, Leighton JL. Org Lett. 2004;6:4375.
-
-
-
For enantioselective Lewis acid catalyzed carbonyl additions of crotylmetal reagents, see: Furuta K, Mouri M, Yamamoto H. Synlett. 1991:561.Yanagasawa A, Ishiba A, Nakashima H, Yamamoto H. Synlett. 1997:88.Yanagasawa A, Kageyama H, Nakatsuka Y, Asakawa K, Matsumoto Y, Yamamoto H. Angew Chem, Int Ed. 1999;38:3701.Aoki S, Mikami K, Terada M, Nakai T. Tetrahedron Lett. 1993;49:1783.Motoyama Y, Okano M, Narusawa H, Makihara N, Aoki K, Nishiyama H. Organometallics. 2001;20:1580.Evans DA, Aye Y, Wu J. Org Lett. 2006;8:2071.Rauniyar V, Hall DG. Angew Chem, Int Ed. 2006;45:2426.Rauniyar V, Zhai H, Hall DG. J Am Chem Soc. 2008;130:8481.
-
-
-
For enantioselective Lewis base catalyzed carbonyl additions of crotylmetal reagents, see: Denmark SE, Coe DM, Pratt NE, Griedel BD. J Org Chem. 1994;59:6161.Denmark SE, Coe DM, Pratt NE, Griedel BD. J Org Chem. 2001;59:6161.Denmark SE, Fu J. J Am Chem Soc. 2001;123:9488.Iseki K, Kuroki Y, Takahashi M, Kishimoto S, Kobayashi Y. Tetrahedron. 1997;53:3513.Nakajima M, Saito M, Shiro M, Hashimoto SI. J Am Chem Soc. 1998;120:6419.Malkov AV, Dufková L, Farrugia L, Kočovsky P. Angew Chem, Int Ed. 2003;42:3674.
-
-
-
For enantioselective chiral diol catalyzed carbonyl additions of crotylmetal reagents, see: Lou S, Moquist PN, Schaus SE. J Am Chem Soc. 2006;128:12660.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

