Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness
- PMID: 19191514
- PMCID: PMC2792127
- DOI: 10.1089/ten.TEA.2008.0451
Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness
Abstract
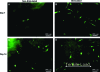
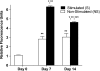
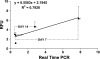
The objectives of this study were to determine how tensile stimulation delivered up to 14 days in culture influenced type I collagen gene expression in stem cells cultured in collagen sponges, and to establish if gene expression, measured using a fluorescence method, correlates with an established method, real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Using a novel model system, mesenchymal stem cells were harvested from six double transgenic mice in which the type I and type II collagen promoters were linked to green fluorescent protein-topaz and enhanced cyan fluorescent protein, respectively. Tissue-engineered constructs were created by seeding 0.5 x 10(6) mesenchymal stem cells onto type I collagen sponge scaffolds in a silicone dish. Constructs were then transferred to a custom pneumatic mechanical stimulation system housed in a standard incubator and stimulated for 5 h=day in tension for either 7 or 14 days using a repeated profile (2.4% peak strain for 20 s at 1 Hz followed by a rest period at 0% strain for 100 s). Control specimens were exposed to identical culture conditions but without mechanical stimulation. At three time points (0, 7, and 14 days), constructs were then prepared for evaluation of gene expression using fluorescence analysis and qRT-PCR, and the remaining constructs were failed in tension. Both analytical methods showed that constructs stimulated for 7 and 14 days showed significantly higher collagen type I gene expression than nonstimulated controls at the same time interval. Gene expression measured using qRT-PCR and fluorescence analysis was positively correlated (r = 0.9). Linear stiffness of stimulated constructs was significantly higher at both 7 and 14 days than that of nonstimulated controls at the same time intervals. Linear stiffness of the stimulated constructs at day 14 was significantly different from that of day 7. Future studies will vary the mechanical signal to optimize type I collagen gene expression to improve construct biomechanics and in vivo tendon repair.
Figures
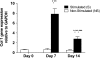

Similar articles
-
Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair.Tissue Eng. 2007 Jun;13(6):1219-26. doi: 10.1089/ten.2006.0339. Tissue Eng. 2007. PMID: 17518715
-
Three-dimensional in vitro effects of compression and time in culture on aggregate modulus and on gene expression and protein content of collagen type II in murine chondrocytes.Tissue Eng Part A. 2009 Oct;15(10):2807-16. doi: 10.1089/ten.TEA.2008.0560. Tissue Eng Part A. 2009. PMID: 19231914 Free PMC article.
-
Mechanical stimulation of tissue engineered tendon constructs: effect of scaffold materials.J Biomech Eng. 2007 Dec;129(6):919-23. doi: 10.1115/1.2800828. J Biomech Eng. 2007. PMID: 18067397
-
Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair.Tissue Eng. 2006 Aug;12(8):2291-300. doi: 10.1089/ten.2006.12.2291. Tissue Eng. 2006. PMID: 16968169
-
Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation.J Orthop Res. 2008 Jan;26(1):1-9. doi: 10.1002/jor.20456. J Orthop Res. 2008. PMID: 17676628 Review.
Cited by
-
Slow stretching that mimics embryonic growth rate stimulates structural and mechanical development of tendon-like tissue in vitro.Dev Dyn. 2011 Nov;240(11):2520-8. doi: 10.1002/dvdy.22760. Dev Dyn. 2011. PMID: 22012594 Free PMC article.
-
The long head of the biceps tendon is a suitable cell source for tendon tissue regeneration.Arch Med Sci. 2014 Jun 29;10(3):587-96. doi: 10.5114/aoms.2014.43752. Epub 2014 Jun 27. Arch Med Sci. 2014. PMID: 25097592 Free PMC article.
-
Pre-hypertrophic chondrogenic enhancer landscape of limb and axial skeleton development.Nat Commun. 2024 Jun 6;15(1):4820. doi: 10.1038/s41467-024-49203-2. Nat Commun. 2024. PMID: 38844479 Free PMC article.
-
Collagen type I and decorin expression in tenocytes depend on the cell isolation method.BMC Musculoskelet Disord. 2012 Aug 8;13:140. doi: 10.1186/1471-2474-13-140. BMC Musculoskelet Disord. 2012. PMID: 22871215 Free PMC article.
-
Effects of cell concentration and collagen concentration on contraction kinetics and mechanical properties in a bone marrow stromal cell-collagen construct.J Biomed Mater Res A. 2010 Jun 1;93(3):1132-9. doi: 10.1002/jbm.a.32606. J Biomed Mater Res A. 2010. PMID: 19768794 Free PMC article.
References
-
- Huang H.H. Qureshi A.A. Biundo J.J., Jr. Sports and other soft tissue injuries, tendinitis, bursitis, and occupation-related syndromes. Curr Opin Rheumatol. 2000;12:150. - PubMed
-
- Liu S. Nguyen T. Ankle sprains and other soft tissue injuries. Curr Opin Rheumatol. 1999;11:132. - PubMed
-
- Bennett W.F. Arthroscopic repair of anterosuperior (supraspinatus/subscapularis) rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Classification of biceps subluxation/instability. Arthroscopy. 2003;19:21. - PubMed
-
- Praemer A. Furner S. Rice D.P. Parke Ridge, IL: American Academy of Orthopaedic Surgeons; 1999. Musculoskeletal Condition in the United States.
-
- Bey M.J. Ramsey M.L. Soslowsky L.J. Intratendinous strain fields of the supraspinatus tendon: effect of a surgically created articular-surface rotator cuff tear. J Shoulder Elbow Surg. 2002;11:562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

