Cytotoxic T-lymphocyte antigen 4 blockade augments the T-cell response primed by attenuated Listeria monocytogenes resulting in more rapid clearance of virulent bacterial challenge
- PMID: 19191906
- PMCID: PMC2753913
- DOI: 10.1111/j.1365-2567.2008.03001.x
Cytotoxic T-lymphocyte antigen 4 blockade augments the T-cell response primed by attenuated Listeria monocytogenes resulting in more rapid clearance of virulent bacterial challenge
Abstract
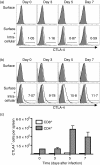
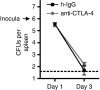
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) uniformly suppresses antigen-specific T cells during chronic infection with bacterial, parasitic or viral pathogens. However, the importance of CTLA-4 in controlling the T-cell response during acute infection or after priming with live attenuated vaccine vectors has not been well characterized. Since strategies aimed at blocking CTLA-4 are being actively developed to therapeutically augment T-cell-mediated immunity, the effects of CTLA-4 blockade on T-cell activation during these conditions need to be more clearly defined. We have examined the role of CTLA-4 in a prime-challenge model of acute bacterial infection using both attenuated and virulent strains of the intracellular bacterium Listeria monocytogenes. Although Foxp3(+) CD4(+) T cells are the predominant CTLA-4-expressing cell type in naïve mice, antigen-specific Foxp3(-) CD4(+) cells upregulate CTLA-4 expression after primary L. monocytogenes infection. Blockade of CTLA-4 results in increased numbers of L. monocytogenes-specific CD4 and CD8 T cells after primary infection with attenuated L. monocytogenes, and confers more rapid bacterial clearance after secondary challenge with virulent L. monocytogenes. Accordingly, CTLA-4 plays an important suppressive role in T-cell priming and protective immunity in a prime-challenge model of acute bacterial infection.
Figures



References
-
- Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY, Bluestone JA, Jenkins MK. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–6. - PubMed
-
- Walsh CM, Smith P, Fallon PG. Role for CTLA-4 but not CD25+ T cells during Schistosoma mansoni infection of mice. Parasite Immunol. 2007;29:293–308. - PubMed
-
- Jacobs T, Graefe SE, Niknafs S, Gaworski I, Fleischer B. Murine malaria is exacerbated by CTLA-4 blockade. J Immunol. 2002;169:2323–9. - PubMed
-
- Lepenies B, Gaworski I, Tartz S, Langhorne J, Fleischer B, Jacobs T. CTLA-4 blockade differentially influences the outcome of non-lethal and lethal Plasmodium yoelii infections. Microbes Infect. 2007;9:687–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

