Mitf dosage as a primary determinant of melanocyte survival after ultraviolet irradiation
- PMID: 19192212
- PMCID: PMC6980044
- DOI: 10.1111/j.1755-148X.2009.00551.x
Mitf dosage as a primary determinant of melanocyte survival after ultraviolet irradiation
Abstract
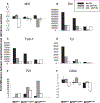
Microphthalmia-associated transcription factor (Mitf) is essential for melanocyte development and function and regulates anti-apoptotic Bcl2 expression. We hypothesized that cellular deficiency of Mitf can influence melanocyte survival in response to ultraviolet (UV) radiation. Primary melanocyte cultures were prepared from neonatal wild-type mice and congenic animals heterozygous for Mitf mutations Mitf (mi-vga9/+) and Mitf(Mi-wh/+) and exposed to UV irradiation. Wild-type melanocytes were more resistant to UV-induced apoptosis than melanocytes partially deficient in Mitf activity, as determined by relative levels of intracellular melanin and relative activation of Mitf target genes Tyr, Tyrp1, Dct, and Cdk2. Comparative experiments with wild-type cells and congenic albino melanocytes demonstrated that these differences are not due to differences in melanin content, implicating Mitf as a primary determinant of UV-dependent melanocyte survival. Mitf activity correlated directly with resistance to UV-induced apoptosis in melanocytes. Mitf was important not only for regulating the expression of anti-apoptotic Bcl-2 following UV irradiation, but also the expression of the pro-apoptotic BH3-only Bad protein and activation of the extrinsic apoptotic pathway. Hence, Mitf is a multifaceted regulator of UV-induced apoptosis in melanocytes.
Figures


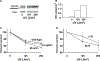
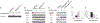
References
-
- Abdel-Malek ZA, Scott MC, Furumura M, Lamoreux ML, Ollmann M, Barsh GS, and Hearing VJ (2001). The melano-cortin 1 receptor is the principal mediator of the effects of agouti signaling protein on mammalian melanocytes. J. Cell Sci 114, 1019–1024. - PubMed
-
- Bivik CA, Andersson EB, and Rosdahl IK (2005). Wavelength-specific effects on UVB-induced apoptosis in melanocytes. A study of Bcl-2/Bax expression and keratinocyte rescue effects. Melanoma Res. 15, 7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

