Rev and Rex proteins of human complex retroviruses function with the MMTV Rem-responsive element
- PMID: 19192308
- PMCID: PMC2661877
- DOI: 10.1186/1742-4690-6-10
Rev and Rex proteins of human complex retroviruses function with the MMTV Rem-responsive element
Abstract
Background: Mouse mammary tumor virus (MMTV) encodes the Rem protein, an HIV Rev-like protein that enhances nuclear export of unspliced viral RNA in rodent cells. We have shown that Rem is expressed from a doubly spliced RNA, typical of complex retroviruses. Several recent reports indicate that MMTV can infect human cells, suggesting that MMTV might interact with human retroviruses, such as human immunodeficiency virus (HIV), human T-cell leukemia virus (HTLV), and human endogenous retrovirus type K (HERV-K). In this report, we test whether the export/regulatory proteins of human complex retroviruses will increase expression from vectors containing the Rem-responsive element (RmRE).
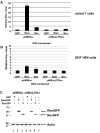
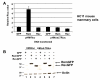
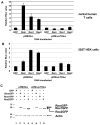
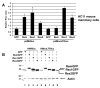
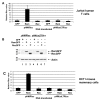
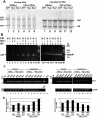
Results: MMTV Rem, HIV Rev, and HTLV Rex proteins, but not HERV-K Rec, enhanced expression from an MMTV-based reporter plasmid in human T cells, and this activity was dependent on the RmRE. No RmRE-dependent reporter gene expression was detectable using Rev, Rex, or Rec in HC11 mouse mammary cells. Cell fractionation and RNA quantitation experiments suggested that the regulatory proteins did not affect RNA stability or nuclear export in the MMTV reporter system. Rem had no demonstrable activity on export elements from HIV, HTLV, or HERV-K. Similar to the Rem-specific activity in rodent cells, the RmRE-dependent functions of Rem, Rev, or Rex in human cells were inhibited by a dominant-negative truncated nucleoporin that acts in the Crm1 pathway of RNA and protein export.
Conclusion: These data argue that many retroviral regulatory proteins recognize similar complex RNA structures, which may depend on the presence of cell-type specific proteins. Retroviral protein activity on the RmRE appears to affect a post-export function of the reporter RNA. Our results provide additional evidence that MMTV is a complex retrovirus with the potential for viral interactions in human cells.
Figures


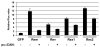
Similar articles
-
Mapping of the functional boundaries and secondary structure of the mouse mammary tumor virus Rem-responsive element.J Biol Chem. 2009 Sep 18;284(38):25642-52. doi: 10.1074/jbc.M109.012476. Epub 2009 Jul 24. J Biol Chem. 2009. PMID: 19632991 Free PMC article.
-
Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus.J Virol. 2005 Dec;79(23):14737-47. doi: 10.1128/JVI.79.23.14737-14747.2005. J Virol. 2005. PMID: 16282474 Free PMC article.
-
Identification of the Rem-responsive element of mouse mammary tumor virus.Nucleic Acids Res. 2008 Nov;36(19):6284-94. doi: 10.1093/nar/gkn608. Epub 2008 Oct 3. Nucleic Acids Res. 2008. PMID: 18835854 Free PMC article.
-
Retroviral RNA elements integrate components of post-transcriptional gene expression.Life Sci. 2001 Oct 26;69(23):2697-709. doi: 10.1016/s0024-3205(01)01360-1. Life Sci. 2001. PMID: 11720075 Review.
-
Nucleocytoplasmic RNA transport in retroviral replication.Results Probl Cell Differ. 2001;34:197-217. doi: 10.1007/978-3-540-40025-7_12. Results Probl Cell Differ. 2001. PMID: 11288676 Review.
Cited by
-
Retroviral Rem protein requires processing by signal peptidase and retrotranslocation for nuclear function.Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12287-92. doi: 10.1073/pnas.1004303107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566871 Free PMC article.
-
Mapping of the functional boundaries and secondary structure of the mouse mammary tumor virus Rem-responsive element.J Biol Chem. 2009 Sep 18;284(38):25642-52. doi: 10.1074/jbc.M109.012476. Epub 2009 Jul 24. J Biol Chem. 2009. PMID: 19632991 Free PMC article.
-
Pericentriolar Targeting of the Mouse Mammary Tumor Virus GAG Protein.PLoS One. 2015 Jun 29;10(6):e0131515. doi: 10.1371/journal.pone.0131515. eCollection 2015. PLoS One. 2015. PMID: 26121257 Free PMC article.
-
A cis-Acting Element Downstream of the Mouse Mammary Tumor Virus Major Splice Donor Critical for RNA Elongation and Stability.J Mol Biol. 2018 Oct 19;430(21):4307-4324. doi: 10.1016/j.jmb.2018.08.025. Epub 2018 Sep 1. J Mol Biol. 2018. PMID: 30179605 Free PMC article.
-
Cross-packaging of genetically distinct mouse and primate retroviral RNAs.Retrovirology. 2009 Jul 14;6:66. doi: 10.1186/1742-4690-6-66. Retrovirology. 2009. PMID: 19602292 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

