A 6-month, double-blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid-cycling bipolar disorder and Co-occurring substance abuse or dependence
- PMID: 19192457
- PMCID: PMC3587136
- DOI: 10.4088/jcp.07m04022
A 6-month, double-blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid-cycling bipolar disorder and Co-occurring substance abuse or dependence
Abstract
Objective: To assess whether combination treatment with lithium and divalproex is more effective than lithium monotherapy in prolonging the time to mood episode recurrence in patients with rapid-cycling bipolar disorder and comorbid substance abuse and/or dependence.
Method: A 6-month, double-blind, parallel-group comparison was carried out in patients who met DSM-IV criteria for (1) bipolar I or II disorder; (2) alcohol, cannabis, or cocaine abuse within the last 3 months or dependence within the last 6 months; (3) rapid cycling during the 12 months preceding study entry; and (4) a history of at least 1 manic, hypomanic, or mixed episode within 3 months of study entry and who had demonstrated a persistent bimodal response to combined treatment with lithium and divalproex. Subjects were randomly assigned to remain on combination treatment or to discontinue divalproex and remain on lithium monotherapy. The study was conducted at an outpatient mood disorders program between October 1997 and October 2006.
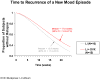
Results: Of 149 patients enrolled into the open-label acute stabilization phase, 79% discontinued prematurely (poor adherence: 42%, nonresponse: 25%, intolerable side effects: 10%). Of 31 patients (21%) randomly assigned to double-blind maintenance treatment, 55% (N = 17) relapsed (24% [N = 4] into depression and 76% [N = 13] into a manic/hypomanic/mixed episode), 26% (N = 8) completed the study, and 19% (N = 6) were poorly adherent or exited prematurely. The median time to recurrence of a new mood episode was 15.9 weeks for patients receiving lithium monotherapy and 17.8 weeks for patients receiving the combination of lithium and divalproex (not significant). The rate of relapse into a mood episode for those receiving lithium monotherapy or the combination of lithium and divalproex was 56% (N = 9) and 53% (N = 8), respectively. The rate of depressive relapse in both arms was 13% (N = 2), while the rate of relapse into a manic, hypomanic, or mixed episode was 44% (N = 7) for lithium monotherapy and 40% (N = 6) for the combination of lithium and divalproex.
Conclusion: A small subgroup of patients in this study stabilized after 6 months of treatment with lithium plus divalproex. Of those who did, the addition of divalproex to lithium conferred no additional prophylactic benefit over lithium alone. Although depression is regarded as the hallmark of rapid-cycling bipolar disorder in general, these data suggest that recurrent episodes of mania tend to be more common in presentations accompanied by comorbid substance use.
Trial registration: clinical trials.gov Identifier: NCT00194129.
Copyright 2009 Physicians Postgraduate Press, Inc.
Figures
Similar articles
-
A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder.Am J Psychiatry. 2005 Nov;162(11):2152-61. doi: 10.1176/appi.ajp.162.11.2152. Am J Psychiatry. 2005. PMID: 16263857 Clinical Trial.
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Lamotrigine as add-on treatment to lithium and divalproex: lessons learned from a double-blind, placebo-controlled trial in rapid-cycling bipolar disorder.Bipolar Disord. 2012 Nov;14(7):780-9. doi: 10.1111/bdi.12013. Bipolar Disord. 2012. PMID: 23107222 Free PMC article. Clinical Trial.
-
A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group.Arch Gen Psychiatry. 2000 May;57(5):481-9. doi: 10.1001/archpsyc.57.5.481. Arch Gen Psychiatry. 2000. PMID: 10807488 Clinical Trial.
-
Bipolar rapid cycling: focus on depression as its hallmark.J Clin Psychiatry. 2001;62 Suppl 14:34-41. J Clin Psychiatry. 2001. PMID: 11469674 Review.
Cited by
-
Comorbid anxiety and substance use disorders associated with a lower use of mood stabilisers in patients with rapid cycling bipolar disorder: a descriptive analysis of the cross-sectional data of 566 patients.Int J Clin Pract. 2010 Feb;64(3):336-44. doi: 10.1111/j.1742-1241.2009.02284.x. Int J Clin Pract. 2010. PMID: 20456174 Free PMC article.
-
The efficacy of valproate in acute mania, bipolar depression and maintenance therapy for bipolar disorder: an overview of systematic reviews with meta-analyses.BMJ Open. 2024 Nov 5;14(11):e087999. doi: 10.1136/bmjopen-2024-087999. BMJ Open. 2024. PMID: 39500601 Free PMC article. Review.
-
Efficacy of pharmacotherapy in bipolar disorder: a report by the WPA section on pharmacopsychiatry.Eur Arch Psychiatry Clin Neurosci. 2012 Jun;262 Suppl 1:1-48. doi: 10.1007/s00406-012-0323-x. Eur Arch Psychiatry Clin Neurosci. 2012. PMID: 22622948
-
Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder.Cochrane Database Syst Rev. 2013 Oct 17;2013(10):CD003196. doi: 10.1002/14651858.CD003196.pub2. Cochrane Database Syst Rev. 2013. PMID: 24132760 Free PMC article.
-
Predictors of non-stabilization during the combination therapy of lithium and divalproex in rapid cycling bipolar disorder: a post-hoc analysis of two studies.Psychopharmacol Bull. 2010;43(1):23-38. Psychopharmacol Bull. 2010. PMID: 20581798 Free PMC article. Clinical Trial.
References
-
- Kupka RW, Luckenbaugh DA, Post RM, Leverich GS, Nolen WA. Rapid and non-rapid cycling bipolar disorder: a meta-analysis of clinical studies. J Clin Psychiatry. 2003;64:1483–94. - PubMed
-
- Schneck CD, Miklowitz DJ, Calabrese JR, et al. Phenomenology of rapid-cycling bipolar disorder: data from the first 500 participants in the Systematic Treatment Enhancement Program. Am J Psychiatry. 2004;161:1902–8. - PubMed
-
- Kupka RW, Luckenbaugh DA, Post RM, et al. Comparison of rapid-cycling and non-rapid-cycling bipolar disorder based on prospective mood ratings in 539 outpatients. Am J Psychiatry. 2005;162:1273–80. - PubMed
-
- Gao K, Bilali S, Conroy C, Ganocy S, Elhaj O, Calabrese JR. Clinical impacts of comorbid anxiety disorder and substance use disorder on patients with rapid cycling bipolar disorder [poster]. Presented at the 159th American Psychiatric Association Annual Meeting; May 20–25, 2006; Toronto, Canada.
-
- Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio RV. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8:232–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

