Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva
- PMID: 19192965
- PMCID: PMC2765577
- DOI: 10.1021/ac802181j
Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva
Abstract
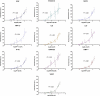
Antibody microarrays have emerged as useful tools for high-throughput protein analysis and candidate biomarker screening. We describe here the development of a multiplexed microsphere-based antibody array capable of simultaneously measuring 10 inflammatory protein mediators. Cytokine-capture microspheres were fabricated by covalently coupling monoclonal antibodies specific for cytokines of interest to fluorescently encoded 3.1 microm polymer microspheres. An optical fiber bundle containing approximately 50,000 individual 3.1 microm diameter fibers was chemically etched to create microwells in which cytokine-capture microspheres could be deposited. Microspheres were randomly distributed in the wells to produce an antibody array for performing a multiplexed sandwich immunoassay. The array responded specifically to recombinant cytokine solutions in a concentration-dependent fashion. The array was also used to examine endogenous mediator patterns in saliva supernatants from patients with pulmonary inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD). This array technology may prove useful as a laboratory-based platform for inflammatory disease research and diagnostics, and its small footprint could also enable integration into a microfluidic cassette for use in point-of-care testing.
Figures






References
-
- Urbanowska T, Mangialaio S, Zickler C, Cheevapruk S, Hasler P, Regenass S, Legay F. J. Immunol. Methods. 2006;316:1–7. - PubMed
-
- Angenendt P. Drug Discov. Today. 2005;10:503–511. - PubMed
-
- Gao W-M, Kuick R, Orchekowski RP, Misek DE, Qiu J, Greenberg AK, Rom WN, Brenner DE, Omenn GS, Haab BB, Hanash SM. BMC Cancer. 2005;5 No pp. given.
-
- Nielsen UB, Geierstanger BH. J. Immunol. Methods. 2004;290:107–120. - PubMed
-
- Holgate ST, Polosa R. Nat. Rev. Immunol. 2008;8:218–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

