Cyclopentadienone iron alcohol complexes: synthesis, reactivity, and implications for the mechanism of iron-catalyzed hydrogenation of aldehydes
- PMID: 19193034
- PMCID: PMC2664094
- DOI: 10.1021/ja808683z
Cyclopentadienone iron alcohol complexes: synthesis, reactivity, and implications for the mechanism of iron-catalyzed hydrogenation of aldehydes
Abstract
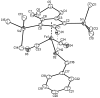
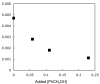
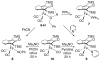
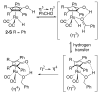
Cyclopentadienone iron alcohol complexes generated from the reactions of [2,5-(SiMe(3))(2)-3,4-(CH(2))(4)(eta(5)-C(4)COH)]Fe(CO)(2)H (3) and aldehydes were characterized by (1)H NMR, (13)C NMR, and IR spectroscopy. The benzyl alcohol complex [2,5-(SiMe(3))(2)-3,4-(CH(2))(4)(eta(4)-C(4)CO)]Fe(CO)(2)(HOCH(2)C(6)H(5)) (6-H) was also characterized by X-ray crystallography. These alcohol complexes are thermally unstable and prone to dissociate the coordinated alcohols. The alcohol ligand is easily replaced by other ligands such as PhCN, pyridine, and PPh(3). Dissociation of the alcohol ligand in the presence of H(2) leads to the formation of iron hydride 3. The reduction of aldehydes by 3 was carried out in the presence of both potential intermolecular and intramolecular traps. The reaction of 3 with PhCHO in the presence of 4-methylbenzyl alcohol as a potential intermolecular trapping agent initially produced only iron complex 6-H of the newly formed benzyl alcohol. However, the reaction of 3 with 4-(HOCD(2))C(6)H(4)CHO, which possesses a potential intramolecular alcohol trapping agent, afforded two alcohol complexes, one with the newly formed alcohol coordinated to iron and the other with the trapping alcohol coordinated. The intramolecular trapping experiments support a mechanism involving concerted transfer of a proton from OH and hydride from Fe of 3 to aldehydes. The kinetics and mechanism of the hydrogenation of benzaldehyde catalyzed by 3 are presented.
Figures


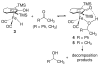
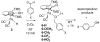
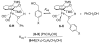
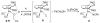
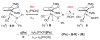

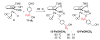
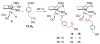
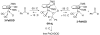

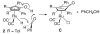

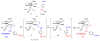
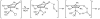
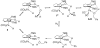
Similar articles
-
Synthesis of carboxylate-bridged iron-thiolate clusters from alcohols/aldehydes or carboxylate salts.Dalton Trans. 2015 Sep 7;44(33):14952-8. doi: 10.1039/c5dt01445j. Dalton Trans. 2015. PMID: 26228059
-
Unexpected direct reduction mechanism for hydrogenation of ketones catalyzed by iron PNP pincer complexes.Inorg Chem. 2011 Dec 19;50(24):12836-43. doi: 10.1021/ic2020176. Epub 2011 Nov 21. Inorg Chem. 2011. PMID: 22103735
-
Mechanistic characterization of aerobic alcohol oxidation catalyzed by Pd(OAc)(2)/pyridine including identification of the catalyst resting state and the origin of nonlinear [catalyst] dependence.J Am Chem Soc. 2004 Sep 15;126(36):11268-78. doi: 10.1021/ja049962m. J Am Chem Soc. 2004. PMID: 15355108
-
Synthesis, spectroscopic characterization, redox properties and catalytic activity of some ruthenium(II) complexes containing aromatic aldehyde and triphenylphosphine or triphenylarsine.Spectrochim Acta A Mol Biomol Spectrosc. 2004 Jan;60(1-2):121-7. doi: 10.1016/s1386-1425(03)00191-4. Spectrochim Acta A Mol Biomol Spectrosc. 2004. PMID: 14670468
-
Hydrosilylation of aldehydes and ketones catalyzed by hydrido iron complexes bearing imine ligands.Dalton Trans. 2014 Aug 14;43(30):11716-22. doi: 10.1039/c4dt00944d. Epub 2014 Jun 23. Dalton Trans. 2014. PMID: 24953036
Cited by
-
Synthesis and reactivity of BINEPINE-based chiral Fe(II) PNP pincer complexes.Monatsh Chem. 2016;147:1023-1030. doi: 10.1007/s00706-016-1706-x. Epub 2016 Mar 21. Monatsh Chem. 2016. PMID: 27340297 Free PMC article.
-
Iron(II)-Catalyzed Aerobic Biomimetic Oxidation of N-Heterocycles.Chemistry. 2021 Oct 1;27(55):13725-13729. doi: 10.1002/chem.202102483. Epub 2021 Sep 6. Chemistry. 2021. PMID: 34324754 Free PMC article.
-
Iron(II)-Catalyzed Aerobic Biomimetic Oxidation of Amines using a Hybrid Hydroquinone/Cobalt Catalyst as Electron Transfer Mediator.Angew Chem Int Ed Engl. 2021 May 17;60(21):11819-11823. doi: 10.1002/anie.202102681. Epub 2021 May 4. Angew Chem Int Ed Engl. 2021. PMID: 33725364 Free PMC article.
-
Substituent Effects and Mechanistic Insights on the Catalytic Activities of (Tetraarylcyclopentadienone)iron Carbonyl Compounds in Transfer Hydrogenations and Dehydrogenations.Organometallics. 2023 Oct 5;42(21):3053-3065. doi: 10.1021/acs.organomet.3c00284. eCollection 2023 Nov 13. Organometallics. 2023. PMID: 38028505 Free PMC article.
-
Iron(II)-Catalyzed Biomimetic Aerobic Oxidation of Alcohols.Angew Chem Int Ed Engl. 2020 Mar 23;59(13):5403-5406. doi: 10.1002/anie.202000054. Epub 2020 Feb 19. Angew Chem Int Ed Engl. 2020. PMID: 31999013 Free PMC article.
References
-
- Sigman MS, Jensen DR. Acc Chem Res. 2006;39:221. - PubMed
-
- Luo XL, Crabtree RH. J Am Chem Soc. 1989;111:2527.
-
- Song JS, Szalda DJ, Bullock RM, Lawrie CJC, Rodkin MA, Norton JR. Angew Chem Int Ed. 1992;31:1233.
- Smith KT, Norton JR, Tilset M. Organometallics. 1996;15:4515.
- Bakhmutov VI, Vorontsov EV, Antonov DY. Inorg Chim Acta. 1998;278:122.
- Bullock RM, Voges MH. J Am Chem Soc. 2000;122:12594.
- Song JS, Szalda DJ, Bullock RM. Organometallics. 2001;20:3337.
- Voges MH, Bullock RM. J Chem Soc Dalton Trans. 2002:759.
-
- Blum Y, Czarkie D, Rahamim Y, Shvo Y. Organometallics. 1985;4:1459.
-
- Casey CP, Bikzhanova GA, Bäckvall JE, Johansson L, Park J, Kim YH. Organometallics. 2002;21:1955.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

