Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis
- PMID: 19193129
- PMCID: PMC2678568
- DOI: 10.1089/ten.tea.2008.0067
Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis
Abstract
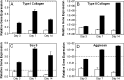
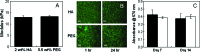
Mesenchymal stem cells (MSCs) are multipotent progenitor cells whose plasticity and self-renewal capacity have generated significant interest for applications in tissue engineering. The objective of this study was to investigate MSC chondrogenesis in photo-cross-linked hyaluronic acid (HA) hydrogels. Because HA is a native component of cartilage, and MSCs may interact with HA via cell surface receptors, these hydrogels could influence stem cell differentiation. In vitro and in vivo cultures of MSC-laden HA hydrogels permitted chondrogenesis, measured by the early gene expression and production of cartilage-specific matrix proteins. For in vivo culture, MSCs were encapsulated with and without transforming growth factor beta-3 (TGF-beta3) or pre-cultured for 2 weeks in chondrogenic medium before implantation. Up-regulation of type II collagen, aggrecan, and sox 9 was observed for all groups over MSCs at the time of encapsulation, and the addition of TGF-beta3 further enhanced the expression of these genes. To assess the influence of scaffold chemistry on chondrogenesis, HA hydrogels were compared with relatively inert poly(ethylene glycol) (PEG) hydrogels and showed enhanced expression of cartilage-specific markers. Differences between HA and PEG hydrogels in vivo were most noticeable for MSCs and polymer alone, indicating that hydrogel chemistry influences the commitment of MSCs to undergo chondrogenesis (e.g., approximately 43-fold up-regulation of type II collagen of MSCs in HA over PEG hydrogels). Although this study investigated only early markers of tissue regeneration, these results emphasize the importance of material cues in MSC differentiation microenvironments, potentially through interactions between scaffold materials and cell surface receptors.
Figures

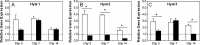





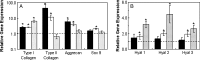
References
-
- Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. - PubMed
-
- Friedenstein A.J. Gorskaja J.F. Kulagina N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267. - PubMed
-
- Alhadlaq A. Mao J.J. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436. - PubMed
-
- Ashton B.A. Allen T.D. Howlett C.R. Eaglesom C.C. Hattori A. Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980:294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

