Functional and complementary phosphorylation state attributes of human insulin-like growth factor-binding protein-1 (IGFBP-1) isoforms resolved by free flow electrophoresis
- PMID: 19193607
- PMCID: PMC2690486
- DOI: 10.1074/mcp.M800571-MCP200
Functional and complementary phosphorylation state attributes of human insulin-like growth factor-binding protein-1 (IGFBP-1) isoforms resolved by free flow electrophoresis
Abstract
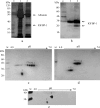
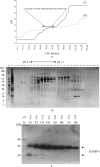
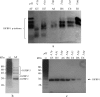
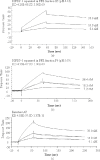
Fetal growth restriction (FGR) is a common disorder in which a fetus is unable to achieve its genetically determined potential size. High concentrations of insulin-like growth factor-binding protein-1 (IGFBP-1) have been associated with FGR. Phosphorylation of IGFBP-1 is a mechanism by which insulin-like growth factor-I (IGF-I) bioavailability can be modulated in FGR. In this study a novel strategy was designed to determine a link between IGF-I affinity and the concomitant phosphorylation state characteristics of IGFBP-1 phosphoisoforms. Using free flow electrophoresis (FFE), multiple IGFBP-1 phosphoisoforms in amniotic fluid were resolved within pH 4.43-5.09. The binding of IGFBP-1 for IGF-I in each FFE fraction was determined with BIAcore biosensor analysis. The IGF-I affinity (K(D)) for different IGFBP-1 isoforms ranged between 1.12e-08 and 4.59e-07. LC-MS/MS characterization revealed four phosphorylation sites, Ser(P)(98), Ser(P)(101), Ser(P)(119), and Ser(P)(169), of which Ser(P)(98) was new. Although the IGF-I binding affinity for IGFBP-1 phosphoisoforms across the FFE fractions did not correlate with phosphopeptide intensities for Ser(P)(101), Ser(P)(98), and Ser(P)(169) sites, a clear association was recorded with Ser(P)(119). Our data demonstrate that phosphorylation at Ser(119) plays a significant role in modulating affinity of IGFBP-1 for IGF-I. In addition, an altered profile of IGFBP-1 phosphoisoforms was revealed between FGR and healthy pregnancies that may result from potential site-specific phosphorylation. This study provides a strong basis for use of this novel approach in establishing the linkage between phosphorylation of IGFBP-1 and FGR. This overall strategy will also be broadly applicable to other phosphoproteins with clinical and functional significance.
Figures


References
-
- Martina, N. A., Kim, E., Chitkara, U., Wathen, N. C., Chard, T., and Giudice, L. C. ( 1997) Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J. Clin. Endocrinol. Metab. 82, 1894–1898 - PubMed
-
- Rajaram, S., Baylink, D. J., and Mohan, S. ( 1997) Insulin-like growth factor-binding proteins in serum and other biological fluids: Regulation and functions. Endocr. Rev. 18, 801–831 - PubMed
-
- Coverley, J. A., and Baxter, R. C. ( 1997) Phosphorylation of insulin-like growth factor binding proteins. Mol. Cell. Endocrinol. 128, 1–5 - PubMed
-
- Gibson, J. M., Aplin, J. D., White, A., and Westwood, M. ( 2001) Regulation of IGF bioavailability in pregnancy. Mol. Hum. Reprod. 7, 79–87 - PubMed
-
- Yu, J., Iwashita, M., Kudo, Y., and Takeda, Y. ( 1998) Phosphorylated insulin-like growth factor (IGF)-binding protein-1 (IGFBP-1) inhibits while non-phosphorylated IGFBP-1 stimulates IGF-I-induced amino acid uptake by cultured trophoblast cells. Growth Horm. IGF Res. 8, 65–70 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

