Effects of cholesteryl ester transfer protein inhibition on apolipoprotein A-II-containing HDL subspecies and apolipoprotein A-II metabolism
- PMID: 19193611
- PMCID: PMC2694343
- DOI: 10.1194/jlr.P800037-JLR200
Effects of cholesteryl ester transfer protein inhibition on apolipoprotein A-II-containing HDL subspecies and apolipoprotein A-II metabolism
Abstract
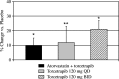
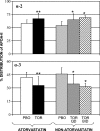
This study was designed to establish the mechanism responsible for the increased apolipoprotein (apo) A-II levels caused by the cholesteryl ester transfer protein inhibitor torcetrapib. Nineteen subjects with low HDL cholesterol (<40 mg/dl), nine of whom were also treated with 20 mg of atorvastatin daily, received placebo for 4 weeks, followed by 120 mg of torcetrapib daily for the next 4 weeks. Six subjects in the nonatorvastatin cohort participated in a third phase, in which they received 120 mg of torcetrapib twice daily for 4 weeks. At the end of each phase, subjects underwent a primed-constant infusion of [5,5,5-(2)H(3)]L-leucine to determine the kinetics of HDL apoA-II. Relative to placebo, torcetrapib significantly increased apoA-II concentrations by reducing HDL apoA-II catabolism in the atorvastatin (-9.4%, P < 0.003) and nonatorvastatin once- (-9.9%, P = 0.02) and twice- (-13.2%, P = 0.02) daily cohorts. Torcetrapib significantly increased the amount of apoA-II in the alpha-2-migrating subpopulation of HDL when given as monotherapy (27%, P < 0.02; 57%, P < 0.003) or on a background of atorvastatin (28%, P < 0.01). In contrast, torcetrapib reduced concentrations of apoA-II in alpha-3-migrating HDL, with mean reductions of -14% (P = 0.23), -18% (P < 0.02), and -18% (P < 0.01) noted during the atorvastatin and nonatorvastatin 120 mg once- and twice-daily phases, respectively. Our findings indicate that CETP inhibition increases plasma concentrations of apoA-II by delaying HDL apoA-II catabolism and significantly alters the remodeling of apoA-II-containing HDL subpopulations.
Figures
Similar articles
-
Cholesteryl Ester Transfer Protein Inhibition With Anacetrapib Decreases Fractional Clearance Rates of High-Density Lipoprotein Apolipoprotein A-I and Plasma Cholesteryl Ester Transfer Protein.Arterioscler Thromb Vasc Biol. 2016 May;36(5):994-1002. doi: 10.1161/ATVBAHA.115.306680. Epub 2016 Mar 10. Arterioscler Thromb Vasc Biol. 2016. PMID: 26966279 Free PMC article. Clinical Trial.
-
Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion.Arterioscler Thromb Vasc Biol. 2005 May;25(5):1057-64. doi: 10.1161/01.ATV.0000161928.16334.dd. Epub 2005 Mar 10. Arterioscler Thromb Vasc Biol. 2005. PMID: 15761191 Free PMC article. Clinical Trial.
-
Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol.N Engl J Med. 2004 Apr 8;350(15):1505-15. doi: 10.1056/NEJMoa031766. N Engl J Med. 2004. PMID: 15071125 Clinical Trial.
-
Effects of cholesteryl ester transfer protein inhibitors on human lipoprotein metabolism: why have they failed in lowering coronary heart disease risk?Curr Opin Lipidol. 2013 Jun;24(3):259-64. doi: 10.1097/MOL.0b013e3283612454. Curr Opin Lipidol. 2013. PMID: 23652567 Review.
-
Increasing high-density lipoprotein cholesterol, inhibition of cholesteryl ester transfer protein, and heart disease risk reduction.Am J Cardiol. 2007 Dec 3;100(11 A):n25-31. doi: 10.1016/j.amjcard.2007.08.010. Am J Cardiol. 2007. PMID: 18047849 Review.
Cited by
-
Cholesteryl Ester Transfer Protein Inhibition With Anacetrapib Decreases Fractional Clearance Rates of High-Density Lipoprotein Apolipoprotein A-I and Plasma Cholesteryl Ester Transfer Protein.Arterioscler Thromb Vasc Biol. 2016 May;36(5):994-1002. doi: 10.1161/ATVBAHA.115.306680. Epub 2016 Mar 10. Arterioscler Thromb Vasc Biol. 2016. PMID: 26966279 Free PMC article. Clinical Trial.
-
High yield expression and purification of recombinant human apolipoprotein A-II in Escherichia coli.J Lipid Res. 2012 Aug;53(8):1708-15. doi: 10.1194/jlr.D028043. Epub 2012 May 25. J Lipid Res. 2012. PMID: 22636422 Free PMC article.
-
A vaccine targeted at CETP alleviates high fat and high cholesterol diet-induced atherosclerosis and non-alcoholic steatohepatitis in rabbit.PLoS One. 2014 Dec 8;9(12):e111529. doi: 10.1371/journal.pone.0111529. eCollection 2014. PLoS One. 2014. PMID: 25486007 Free PMC article.
-
Does Elevated High-Density Lipoprotein Cholesterol Protect Against Cardiovascular Disease?J Clin Endocrinol Metab. 2024 Jan 18;109(2):321-332. doi: 10.1210/clinem/dgad406. J Clin Endocrinol Metab. 2024. PMID: 37437107 Free PMC article.
-
Understanding HDL Metabolism and Biology Through In Vivo Tracer Kinetics.Arterioscler Thromb Vasc Biol. 2024 Jan;44(1):76-88. doi: 10.1161/ATVBAHA.123.319742. Epub 2023 Nov 30. Arterioscler Thromb Vasc Biol. 2024. PMID: 38031838 Free PMC article. Review.
References
-
- Cheung M. C., and J. J. Albers. 1982. Distribution of high density lipoprotein particles with different apoprotein composition: particles with apoA-I and apoA-I and particles with apoA-I but not apoA-II. J. Lipid Res. 23 747–753. - PubMed
-
- Massey J. B., M. F. Rohde, W. B. Van Winkle, A. M. Gotto, Jr., and H. J. Pownall. 1981. Physical properties of lipid-protein complexes formed by the interaction of dimyristoylphosphatidylcholine and human high-density apolipoprotein A-II. Biochemistry. 20 1569–1574. - PubMed
-
- Rye K-A., and P. J. Barter. 1994. The influence of apolipoproteins on the structure and function of spheroidal, reconstituted high-density lipoproteins. J. Biol. Chem. 269 10298–10303. - PubMed
-
- Broedl U. C., W. Jin, I. V. Fuki, J. S. Millar, and D. J. Rader. 2006. Endothelial lipase is less effective at influencing HDL metabolism in vivo in mice expressing apoA-II. J. Lipid Res. 47 2191–2197. - PubMed
-
- Lagrost L., L. Persegol, C. Lallemant, and P. Gambert. 1994. Influence of apolipoprotein composition of high density lipoprotein particles on cholesteryl ester transfer protein activity. J. Biol. Chem. 269 3189–3197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

