Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity
- PMID: 19193623
- PMCID: PMC2646557
- DOI: 10.1083/jcb.200808130
Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity
Abstract
Cells have evolved a signaling pathway called the spindle assembly checkpoint (SAC) to increase the fidelity of chromosome segregation by generating a "wait anaphase" signal until all chromosomes are properly aligned within the mitotic spindle. It has been proposed that tension generated by the stretch of the centromeric chromatin of bioriented chromosomes stabilizes kinetochore microtubule attachments and turns off SAC activity. Although biorientation clearly causes stretching of the centromeric chromatin, it is unclear whether the kinetochore is also stretched. To test whether intrakinetochore stretch occurs and is involved in SAC regulation, we developed a Drosophila melanogaster S2 cell line expressing centromere identifier-mCherry and Ndc80-green fluorescent protein to mark the inner and outer kinetochore domains, respectively. We observed stretching within kinetochores of bioriented chromosomes by monitoring both inter- and intrakinetochore distances in live cell assays. This intrakinetochore stretch is largely independent of a 30-fold variation in centromere stretch. Furthermore, loss of intrakinetochore stretch is associated with enhancement of 3F3/2 phosphorylation and SAC activation.
Figures
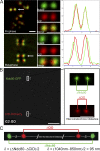
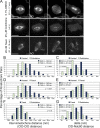
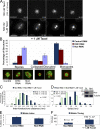
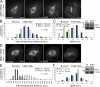

Comment in
-
Releasing the spindle assembly checkpoint without tension.J Cell Biol. 2009 Feb 9;184(3):355-6. doi: 10.1083/jcb.200812016. Epub 2009 Feb 3. J Cell Biol. 2009. PMID: 19193624 Free PMC article.
References
-
- Basto R., Gomes R., Karess R.E. 2000. Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila.Nat. Cell Biol. 2:939–943 - PubMed
-
- Buffin E., Lefebvre C., Huang J., Gagou M.E., Karess R.E. 2005. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex.Curr. Biol. 15:856–861 - PubMed
-
- Famulski J.K., Chan G.K. 2007. Aurora B kinase-dependent recruitment of hZW10 and hROD to tensionless kinetochores.Curr. Biol. 17:2143–2149 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

