Aquaporin-2 in the "-omics" era
- PMID: 19193633
- PMCID: PMC2685649
- DOI: 10.1074/jbc.R900006200
Aquaporin-2 in the "-omics" era
Abstract
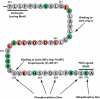
Vasopressin controls renal water excretion largely through actions to regulate the water channel aquaporin-2 in collecting duct principal cells. Our knowledge of the mechanisms involved has increased markedly in recent years with the advent of methods for large-scale systems-level profiling such as protein mass spectrometry, yeast two-hybrid analysis, and oligonucleotide microarrays. Here we review this progress.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

