Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype
- PMID: 19193641
- PMCID: PMC2666557
- DOI: 10.1074/jbc.M806989200
Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype
Abstract
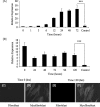
The differentiation of resident fibroblasts to myofibroblasts is central to wound healing. In the context of organ fibrosis, however, persistence of these myofibroblasts is associated with progressive disease. This study examines mechanisms controlling the maintenance of the myofibroblast phenotype. Myofibroblasts were induced by adding transforming growth factor-beta1 (TGF-beta1) (10 ng/ml) to fibroblasts for 72 h. The phenotype was maintained for up to 120 h following removal of TGF-beta1. Western blot for pSmad2 and -3 demonstrated persistent phosphorylation despite removal of exogenous TGF-beta1. This persistence was because of autocrine synthesis of TGF-beta1, which was inhibited by both anti-TGF-beta1 antibody and the ALK5 inhibitor SB431542. Persistence of phenotype was also associated with increased hyaluronan (HA) generation, synthesis of the hyaladherin TSG6, and HA pericellular coat formation. These were all inhibited by TGF-beta receptor blockade. To further investigate the importance of HA synthesis, 4-methylumbelliferone was used to deplete the cytoplasmic pool of UDP-glucuronic acid, essential for HA chain elongation. This prevented formation of the pericellular HA matrix and decreased expression of alpha-SMA. 4-Methylumbelliferone had no effect, however, on Smad2 and -3 phosphorylation. Similarly inhibition of HAS2 by short interfering RNA prevented phenotypic activation without altering TGF-beta1-dependent Smad phosphorylation, thus suggesting that HA-dependent regulation of cell phenotype was independent of Smad activation. These data suggest that myofibroblasts in areas of fibrosis maintain their own phenotype through autocrine TGF-beta1 action and that extracellular HA matrices are an essential mediator of this. We propose a model in which the formation of the pericellular HA matrix regulates the outcome of Smad-dependent autocrine TGF-beta1-activated signaling, and therefore persistence of the myofibroblast phenotype.
Figures




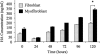

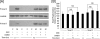

Similar articles
-
Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation.Am J Pathol. 2009 Nov;175(5):1915-28. doi: 10.2353/ajpath.2009.090045. Epub 2009 Oct 1. Am J Pathol. 2009. PMID: 19808648 Free PMC article.
-
Dysfunctional pericellular hyaluronan deposition contributes to attenuated CD44/EGFR co-localization and impaired myofibroblast differentiation in chronic wound fibroblasts.Exp Cell Res. 2025 Jul 15;450(2):114646. doi: 10.1016/j.yexcr.2025.114646. Epub 2025 Jun 14. Exp Cell Res. 2025. PMID: 40523596
-
Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts.J Biol Chem. 2013 May 24;288(21):14824-38. doi: 10.1074/jbc.M113.451336. Epub 2013 Apr 15. J Biol Chem. 2013. PMID: 23589287 Free PMC article.
-
The hard problem: Mechanotransduction perpetuates the myofibroblast phenotype in scleroderma fibrosis.Wound Repair Regen. 2021 Jul;29(4):582-587. doi: 10.1111/wrr.12889. Epub 2021 Jan 7. Wound Repair Regen. 2021. PMID: 33410577 Review.
-
Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis.Biomolecules. 2021 Jul 23;11(8):1095. doi: 10.3390/biom11081095. Biomolecules. 2021. PMID: 34439762 Free PMC article. Review.
Cited by
-
The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium.J Cardiovasc Transl Res. 2012 Dec;5(6):837-47. doi: 10.1007/s12265-012-9406-3. Epub 2012 Sep 7. J Cardiovasc Transl Res. 2012. PMID: 22956156 Free PMC article. Review.
-
TSG6 hyaluronan matrix remodeling dampens the inflammatory response during colitis.Matrix Biol. 2023 Aug;121:149-166. doi: 10.1016/j.matbio.2023.06.007. Epub 2023 Jun 29. Matrix Biol. 2023. PMID: 37391162 Free PMC article.
-
Attenuation of Radiation-Induced Lung Injury by Hyaluronic Acid Nanoparticles.Front Pharmacol. 2020 Aug 12;11:1199. doi: 10.3389/fphar.2020.01199. eCollection 2020. Front Pharmacol. 2020. PMID: 32903478 Free PMC article.
-
A Role for HAPLN1 During Phenotypic Modulation of Human Lung Fibroblasts In Vitro.J Histochem Cytochem. 2020 Nov;68(11):797-811. doi: 10.1369/0022155420966663. Epub 2020 Oct 16. J Histochem Cytochem. 2020. PMID: 33064036 Free PMC article.
-
A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds.Am J Pathol. 2012 Oct;181(4):1250-70. doi: 10.1016/j.ajpath.2012.06.036. Epub 2012 Aug 11. Am J Pathol. 2012. PMID: 22889846 Free PMC article.
References
-
- Sappino, A. P., Schürch, W., and Gabbiani, G. (1990) Lab. Investig. 63 144–161 - PubMed
-
- Gabbiani, G. (2003) J. Pathol. 200 500–503 - PubMed
-
- Desmouliere, A., and Gabbiani, G. (1996) in The Molecular and Cellular Biology of Wound Repair (Clark, R. A. F., ed) 2nd Ed., pp. 391–423, Plenum Publishing Corp., New York
-
- Essawy, M., Soylemezoglu, O., Muchaneta-Kubara, E. C., Shortland, J., Brown, C. B., and El Nahas, A. M. (1997) Nephrol. Dial. Transplant. 12 43–50 - PubMed
-
- Goumenos, D. S., Brown, C. B., Shortland, J., and El Nahas, A. M. (1994) Nephrol. Dial. Transplant. 9 1418–1425 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

