NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti
- PMID: 19193723
- PMCID: PMC2670640
- DOI: 10.1152/ajprenal.90564.2008
NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti
Abstract
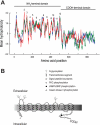
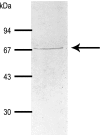
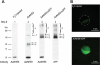
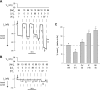
The goal of this study was to identify and characterize the hypothesized apical cation/H(+) exchanger responsible for K(+) and/or Na(+) secretion in the renal (Malpighian) tubules of the yellow fever mosquito Aedes aegypti. From Aedes Malpighian tubules, we cloned "AeNHE8," a full-length cDNA encoding an ortholog of mammalian Na(+)/H(+) exchanger 8 (NHE8). The expression of AeNHE8 transcripts is ubiquitous among mosquito tissues and is not enriched in Malpighian tubules. Western blots of Malpighian tubules suggest that AeNHE8 is expressed primarily as an intracellular protein, which was confirmed by immunohistochemical localizations in Malpighian tubules. AeNHE8 immunoreactivity is expressed in principal cells of the secretory, distal segments, where it localizes to a subapical compartment (e.g., vesicles or endosomes), but not in the apical brush border. Furthermore, feeding mosquitoes a blood meal or treating isolated tubules with dibutyryl-cAMP, both of which stimulate a natriuresis by Malpighian tubules, do not influence the intracellular localization of AeNHE8 in principal cells. When expressed heterologously in Xenopus laevis oocytes, AeNHE8 mediates EIPA-sensitive Na/H exchange, in which Li(+) partially and K(+) poorly replace Na(+). The expression of AeNHE8 in Xenopus oocytes is associated with the development of a conductive pathway that closely resembles the known endogenous nonselective cation conductances of Xenopus oocytes. In conclusion, AeNHE8 does not mediate cation/H(+) exchange in the apical membrane of Aedes Malpighian tubules; it is more likely involved with an intracellular function.
Figures









Similar articles
-
Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti.Acta Physiol (Oxf). 2011 Jul;202(3):387-407. doi: 10.1111/j.1748-1716.2010.02195.x. Epub 2010 Nov 16. Acta Physiol (Oxf). 2011. PMID: 20946239 Free PMC article. Review.
-
Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti.Insect Biochem Mol Biol. 2013 Jan;43(1):75-90. doi: 10.1016/j.ibmb.2012.09.009. Epub 2012 Oct 17. Insect Biochem Mol Biol. 2013. PMID: 23085358 Free PMC article.
-
NHE8 mediates amiloride-sensitive Na+/H+ exchange across mosquito Malpighian tubules and catalyzes Na+ and K+ transport in reconstituted proteoliposomes.Am J Physiol Renal Physiol. 2007 May;292(5):F1501-12. doi: 10.1152/ajprenal.00487.2005. Epub 2007 Feb 6. Am J Physiol Renal Physiol. 2007. PMID: 17287198
-
Role of an apical K,Cl cotransporter in urine formation by renal tubules of the yellow fever mosquito (Aedes aegypti).Am J Physiol Regul Integr Comp Physiol. 2011 Nov;301(5):R1318-37. doi: 10.1152/ajpregu.00223.2011. Epub 2011 Aug 3. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21813871 Free PMC article.
-
NHE(VNAT): an H+ V-ATPase electrically coupled to a Na+:nutrient amino acid transporter (NAT) forms an Na+/H+ exchanger (NHE).J Exp Biol. 2009 Feb;212(Pt 3):347-57. doi: 10.1242/jeb.026047. J Exp Biol. 2009. PMID: 19151209 Free PMC article. Review.
Cited by
-
Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti.Acta Physiol (Oxf). 2011 Jul;202(3):387-407. doi: 10.1111/j.1748-1716.2010.02195.x. Epub 2010 Nov 16. Acta Physiol (Oxf). 2011. PMID: 20946239 Free PMC article. Review.
-
A SLC4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): evidence for a novel role of stellate cells in diuretic fluid secretion.Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R642-60. doi: 10.1152/ajpregu.00729.2009. Epub 2009 Dec 30. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20042685 Free PMC article.
-
Pharmacological validation of an inward-rectifier potassium (Kir) channel as an insecticide target in the yellow fever mosquito Aedes aegypti.PLoS One. 2014 Jun 24;9(6):e100700. doi: 10.1371/journal.pone.0100700. eCollection 2014. PLoS One. 2014. PMID: 24959745 Free PMC article.
-
Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti.Insect Biochem Mol Biol. 2013 Jan;43(1):75-90. doi: 10.1016/j.ibmb.2012.09.009. Epub 2012 Oct 17. Insect Biochem Mol Biol. 2013. PMID: 23085358 Free PMC article.
-
The GPA-dependent, spherostomatocytosis mutant AE1 E758K induces GPA-independent, endogenous cation transport in amphibian oocytes.Am J Physiol Cell Physiol. 2010 Feb;298(2):C283-97. doi: 10.1152/ajpcell.00444.2009. Epub 2009 Nov 11. Am J Physiol Cell Physiol. 2010. PMID: 19907019 Free PMC article.
References
-
- Ahearn GA, Clay LP. Kinetic analysis of electrogenic 2Na+-1H+ antiport in crustacean hepatopancreas. Am J Physiol Regul Integr Comp Physiol 257: R484–R493, 1989. - PubMed
-
- Ahearn GA, Franco P. Electrogenic 2Na+/H+ antiport in Echinoderm gastrointestinal epithelium. J Exp Biol 158: 495–508, 1991. - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795, 2004. - PubMed
-
- Beyenbach KW Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci 16: 145–151, 2001. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

