Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques
- PMID: 19193759
- PMCID: PMC7051670
- DOI: 10.3174/ajnr.A1477
Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques
Abstract
Background and purpose: Traumatic brain injury (TBI) is a classic model of monophasic neuronal and axonal injury, in which tissue damage mainly occurs at the moment of trauma. There is some evidence of delayed progression of the neuronal and axonal loss. Our purpose was to test the hypothesis that quantitative MR imaging techniques can estimate the biologic changes secondary to delayed neuronal and axonal loss after TBI.
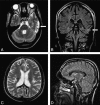
Materials and methods: Nine patients (age, 11-28 years; 5 male) who sustained a moderate or severe TBI were evaluated at a mean of 3.1 years after trauma. We applied the following techniques: bicaudate (BCR) and bifrontal (BFR) ventricle-to-brain ratios; T2 relaxometry; magnetization transfer ratio (MTR); apparent diffusion coefficient (ADC); and proton spectroscopy, by using an N-acetylaspartate/creatine (NAA/Cr) ratio measured in normal-appearing white matter (NAWM) and the corpus callosum (CC). The results were compared with those of a control group.
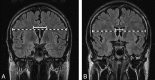
Results: BCR and BFR mean values were significantly increased (P < or = .05) in patients due to secondary subcortical atrophy; increased T2 relaxation time was observed in the NAWM and CC, reflecting an increase in water concentration secondary to axonal loss. Increased ADC mean values and reduced MTR mean values were found in the NAWM and CC, showing damage in the myelinated axonal fibers; and decreased NAA/Cr ratio mean values were found in the CC, indicating axonal loss.
Conclusions: These quantitative MR imaging techniques could noninvasively demonstrate the neuronal and axonal damage in the NAWM and CC of human brains, secondary to moderate or severe TBI.
Figures


Similar articles
-
Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: correlation with clinical outcome after traumatic brain injury.AJNR Am J Neuroradiol. 2001 Jan;22(1):143-51. AJNR Am J Neuroradiol. 2001. PMID: 11158900 Free PMC article.
-
Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients.J Neurosurg. 1998 May;88(5):795-801. doi: 10.3171/jns.1998.88.5.0795. J Neurosurg. 1998. PMID: 9576245
-
Corpus callosum axonal injury in multiple sclerosis measured by proton magnetic resonance spectroscopic imaging.Arch Neurol. 2004 Jul;61(7):1081-6. doi: 10.1001/archneur.61.7.1081. Arch Neurol. 2004. PMID: 15262739
-
White matter involvement after TBI: Clues to axon and myelin repair capacity.Exp Neurol. 2016 Jan;275 Pt 3:328-333. doi: 10.1016/j.expneurol.2015.02.011. Epub 2015 Feb 16. Exp Neurol. 2016. PMID: 25697845 Review.
-
Characterization of tissue damage in multiple sclerosis by nuclear magnetic resonance.Philos Trans R Soc Lond B Biol Sci. 1999 Oct 29;354(1390):1675-86. doi: 10.1098/rstb.1999.0511. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10603619 Free PMC article. Review.
Cited by
-
Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study.J Neurotrauma. 2011 Oct;28(10):2091-102. doi: 10.1089/neu.2010.1739. Epub 2011 Sep 29. J Neurotrauma. 2011. PMID: 21761962 Free PMC article.
-
Fornix Integrity Is Differently Associated With Cognition in Healthy Aging and Non-amnestic Mild Cognitive Impairment: A Pilot Diffusion Tensor Imaging Study in Thai Older Adults.Front Aging Neurosci. 2020 Dec 2;12:594002. doi: 10.3389/fnagi.2020.594002. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33343334 Free PMC article.
-
Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury.J Cereb Blood Flow Metab. 2013 Sep;33(9):1474-84. doi: 10.1038/jcbfm.2013.108. Epub 2013 Jun 26. J Cereb Blood Flow Metab. 2013. PMID: 23801244 Free PMC article.
-
Application of Synthetic MRI for Direct Measurement of Magnetic Resonance Relaxation Time and Tumor Volume at Multiple Time Points after Contrast Administration: Preliminary Results in Patients with Brain Metastasis.Korean J Radiol. 2018 Jul-Aug;19(4):783-791. doi: 10.3348/kjr.2018.19.4.783. Epub 2018 Jun 14. Korean J Radiol. 2018. PMID: 29962885 Free PMC article.
-
The Delayed Neuroprotective Effect of Methylene Blue in Experimental Rat Brain Trauma.Antioxidants (Basel). 2020 May 2;9(5):377. doi: 10.3390/antiox9050377. Antioxidants (Basel). 2020. PMID: 32370131 Free PMC article.
References
-
- Smith DH, Chen XH, Pierce JE, et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma 1997;14:715–27 - PubMed
-
- Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol 2002;103:607–14 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
