Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites
- PMID: 19193765
- PMCID: PMC2663929
- DOI: 10.1091/mbc.e08-09-0943
Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites
Abstract
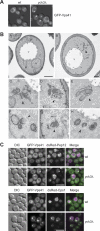
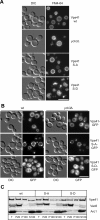
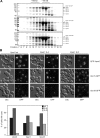
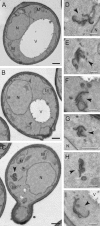
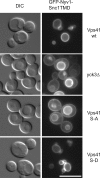
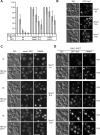
Membrane fusion depends on multisubunit tethering factors such as the vacuolar HOPS complex. We previously showed that the vacuolar casein kinase Yck3 regulates vacuole biogenesis via phosphorylation of the HOPS subunit Vps41. Here, we link the identified Vps41 phosphorylation site to HOPS function at the endosome-vacuole fusion site. The nonphosphorylated Vps41 mutant (Vps41 S-A) accumulates together with other HOPS subunits on punctate structures proximal to the vacuole that expand in a class E mutant background and that correspond to in vivo fusion sites. Ultrastructural analysis of this mutant confirmed the presence of tubular endosomal structures close to the vacuole. In contrast, Vps41 with a phosphomimetic mutation (Vps41 S-D) is mislocalized and leads to multilobed vacuoles, indicative of a fusion defect. These two phenotypes can be rescued by overproduction of the vacuolar Rab Ypt7, revealing that both Ypt7 and Yck3-mediated phosphorylation modulate the Vps41 localization to the endosome-vacuole junction. Our data suggest that Vps41 phosphorylation fine-tunes the organization of vacuole fusion sites and provide evidence for a fusion "hot spot" on the vacuole limiting membrane.
Figures


References
-
- Albert S., Gallwitz D. Two new members of a family of Ypt/Rab GTPase activating proteins. Promiscuity of substrate recognition. J. Biol. Chem. 1999;274:33186–33189. - PubMed
-
- Bos J. L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

