Attentional modulation of visual responses by flexible input gain
- PMID: 19193776
- PMCID: PMC2695627
- DOI: 10.1152/jn.90654.2008
Attentional modulation of visual responses by flexible input gain
Abstract
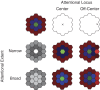
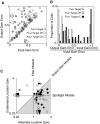
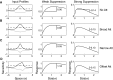
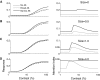
Although it is clear that sensory responses in the cortex can be strongly modulated by stimuli outside of classical receptive fields as well as by extraretinal signals such as attention and anticipation, the exact rules governing the neuronal integration of sensory and behavioral signals remain unclear. For example, most experiments studying sensory interactions have not explored attention, while most studies of attention have relied on the responses to relatively limited sets of stimuli. However, a recent study of V4 responses, in which location, orientation, and spatial attention were systematically varied, suggests that attention can both facilitate and suppress specific sensory inputs to a neuron according to behavioral relevance. To explore the implications of such input gain, we modeled the effects of a center-surround organization of attentional modulation using existing receptive field models of sensory integration. The model is consistent with behavioral measurements of a suppressive effect that surrounds the facilitatory locus of spatial attention. When this center-surround modulation is incorporated into realistic models of sensory integration, it is able to explain seemingly disparate observations of attentional effects in the neurophysiological literature, including spatial shifts in receptive field position and the preferential modulation of low contrast stimuli. The model is also consistent with recent formulations of attention to features in which gain is variably applied among cells with different receptive field properties. Consistent with functional imaging results, the model predicts that spatial attention effects will vary between different visual areas and suggests that attention may act through a common mechanism of selective and flexible gain throughout the visual system.
Figures



Similar articles
-
V4 receptive field dynamics as predicted by a systems-level model of visual attention using feedback from the frontal eye field.Neural Netw. 2006 Nov;19(9):1371-82. doi: 10.1016/j.neunet.2006.08.006. Epub 2006 Oct 2. Neural Netw. 2006. PMID: 17014990
-
Modulation of sensory suppression: implications for receptive field sizes in the human visual cortex.J Neurophysiol. 2001 Sep;86(3):1398-411. doi: 10.1152/jn.2001.86.3.1398. J Neurophysiol. 2001. PMID: 11535686
-
Spatial summation can explain the attentional modulation of neuronal responses to multiple stimuli in area V4.J Neurosci. 2008 May 7;28(19):5115-26. doi: 10.1523/JNEUROSCI.0138-08.2008. J Neurosci. 2008. PMID: 18463265 Free PMC article.
-
Surround suppression supports second-order feature encoding by macaque V1 and V2 neurons.Vision Res. 2014 Nov;104:24-35. doi: 10.1016/j.visres.2014.10.004. Epub 2014 Oct 23. Vision Res. 2014. PMID: 25449336 Free PMC article. Review.
-
Visual streams and shifting attention.Prog Brain Res. 2009;176:47-63. doi: 10.1016/S0079-6123(09)17604-5. Prog Brain Res. 2009. PMID: 19733749 Review.
Cited by
-
Neural dynamics of object-based multifocal visual spatial attention and priming: object cueing, useful-field-of-view, and crowding.Cogn Psychol. 2012 Aug;65(1):77-117. doi: 10.1016/j.cogpsych.2012.02.001. Epub 2012 Mar 14. Cogn Psychol. 2012. PMID: 22425615 Free PMC article.
-
An Extended Normalization Model of Attention Accounts for Feature-Based Attentional Enhancement of Both Response and Coherence Gain.PLoS Comput Biol. 2016 Dec 15;12(12):e1005225. doi: 10.1371/journal.pcbi.1005225. eCollection 2016 Dec. PLoS Comput Biol. 2016. PMID: 27977679 Free PMC article.
-
Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1.Neuron. 2010 Apr 15;66(1):114-25. doi: 10.1016/j.neuron.2010.03.013. Neuron. 2010. PMID: 20399733 Free PMC article.
-
Dynamic changes in superior temporal sulcus connectivity during perception of noisy audiovisual speech.J Neurosci. 2011 Feb 2;31(5):1704-14. doi: 10.1523/JNEUROSCI.4853-10.2011. J Neurosci. 2011. PMID: 21289179 Free PMC article.
-
Attention operates uniformly throughout the classical receptive field and the surround.Elife. 2016 Aug 22;5:e17256. doi: 10.7554/eLife.17256. Elife. 2016. PMID: 27547989 Free PMC article.
References
-
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci 10: 124–130, 2006. - PubMed
-
- Born R, Tootell R. Segregation of global and local motion processing in primate middle temporal visual area [published erratum appears in Nature 365: 279, et al. 1993]. Nature 357: 497–499, 1992. - PubMed
-
- Boudreau CE, Williford TH, Maunsell JHR. Effects of task difficulty and target likelihood in area v4 of macaque monkeys. J Neurophysiol 96: 2377–2387, 2006. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

