HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium
- PMID: 19193779
- PMCID: PMC2653690
- DOI: 10.1681/ASN.2008040424
HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium
Abstract
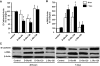
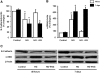
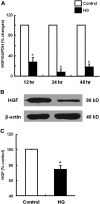
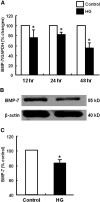
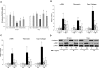
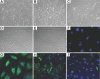
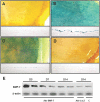
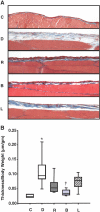
Over time, peritoneal dialysis results in functional and structural alterations of the peritoneal membrane, but the underlying mechanisms and whether these changes are reversible are not completely understood. Here, we studied the effects of high levels of glucose, which are found in the dialysate, on human peritoneal mesothelial cells (HPMCs). We found that high concentrations of glucose induced epithelial-to-mesenchymal transition (EMT) of HPMC, suggested by decreased expression of E-cadherin and increased expression of alpha-smooth muscle actin, fibronectin, and type I collagen and by increased cell migration. Normalization of glucose concentration on day 2 reversed the phenotypic transformation, but the changes were irreversible after 7 d of stimulation with high glucose. In addition, exposure of HPMC to high glucose resulted in a decreased expression of the antifibrotic cytokines, hepatocyte growth factor (HGF) and bone morphogenic protein 7 (BMP-7). Exogenous treatment with HGF resulted in a dosage-dependent prevention of high glucose-induced EMT. Both BMP-7 peptide and gene transfection with an adenoviral vector of BMP-7 also protected HPMCs from EMT. Furthermore, adenoviral BMP-7 transfection decreased peritoneal EMT and ameliorated peritoneal thickening in an animal model of peritoneal dialysis. In summary, high concentrations of glucose induce a reversible EMT of HPMCs, associated with decreased production of HGF and BMP-7. Treatment of HPMCs with HGF or BMP-7 blocks high glucose-induced EMT, and BMP-7 ameliorates peritoneal fibrosis in an animal model of peritoneal dialysis.
Figures
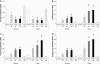


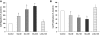

Similar articles
-
BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure.Nephrol Dial Transplant. 2010 Apr;25(4):1098-108. doi: 10.1093/ndt/gfp618. Epub 2010 Jan 12. Nephrol Dial Transplant. 2010. PMID: 20067910
-
The effect of statin on epithelial-mesenchymal transition in peritoneal mesothelial cells.PLoS One. 2014 Oct 2;9(10):e109628. doi: 10.1371/journal.pone.0109628. eCollection 2014. PLoS One. 2014. PMID: 25275561 Free PMC article.
-
Paricalcitol ameliorates epithelial-to-mesenchymal transition in the peritoneal mesothelium.Nephron Exp Nephrol. 2014;126(1):1-7. doi: 10.1159/000357156. Epub 2014 Jan 17. Nephron Exp Nephrol. 2014. PMID: 24458092
-
Mechanisms of epithelial-mesenchymal transition of peritoneal mesothelial cells during peritoneal dialysis.J Korean Med Sci. 2007 Dec;22(6):943-5. doi: 10.3346/jkms.2007.22.6.943. J Korean Med Sci. 2007. PMID: 18162703 Free PMC article. Review.
-
Effect of high glucose on peritoneal mesothelial cell biology.Perit Dial Int. 2000;20 Suppl 2:S15-8. Perit Dial Int. 2000. PMID: 10911637 Review.
Cited by
-
Modulation of the cancer cell transcriptome by culture media formulations and cell density.Int J Oncol. 2015 May;46(5):2067-75. doi: 10.3892/ijo.2015.2930. Epub 2015 Mar 17. Int J Oncol. 2015. PMID: 25776572 Free PMC article.
-
Pathophysiological Mechanisms of Peritoneal Fibrosis and Peritoneal Membrane Dysfunction in Peritoneal Dialysis.Int J Mol Sci. 2024 Aug 7;25(16):8607. doi: 10.3390/ijms25168607. Int J Mol Sci. 2024. PMID: 39201294 Free PMC article. Review.
-
Are the Mesothelial-to-Mesenchymal Transition, Sclerotic Peritonitis Syndromes, and Encapsulating Peritoneal Sclerosis Part of the Same Process?Int J Nephrol. 2013;2013:263285. doi: 10.1155/2013/263285. Epub 2013 Feb 10. Int J Nephrol. 2013. PMID: 23476771 Free PMC article.
-
Vitamin D3 alleviates lung fibrosis of type 2 diabetic rats via SIRT3 mediated suppression of pyroptosis.Apoptosis. 2023 Dec;28(11-12):1618-1627. doi: 10.1007/s10495-023-01878-6. Epub 2023 Aug 2. Apoptosis. 2023. PMID: 37530936
-
Network-based integrated analysis of omics data reveal novel players of TGF-β1-induced EMT in human peritoneal mesothelial cells.Sci Rep. 2019 Feb 6;9(1):1497. doi: 10.1038/s41598-018-37101-9. Sci Rep. 2019. PMID: 30728376 Free PMC article.
References
-
- Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI: What really happens to people on long-term peritoneal dialysis? Kidney Int 54: 2207–2217, 1998 - PubMed
-
- Krediet RT: The peritoneal membrane in chronic peritoneal dialysis. Kidney Int 55: 341–356, 1999 - PubMed
-
- Davies SJ, Phillips L, Naish PF, Russell GI: Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol 12: 1046–1051, 2001 - PubMed
-
- Holmes CJ, Faict D: Peritoneal dialysis solution biocompatibility: Definitions and evaluation strategies. Kidney Int Suppl 64: S50–S56, 2003 - PubMed
-
- Cendoroglo M, Sundaram S, Jaber BL, Pereira BJ: Effect of glucose concentration, osmolality, and sterilization process of peritoneal dialysis fluids on cytokine production by peripheral blood mononuclear cells and polymorphonuclear cell functions in vitro. Am J Kidney Dis 31: 273–282, 1998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

