Human rhinovirus type 2 uncoating at the plasma membrane is not affected by a pH gradient but is affected by the membrane potential
- PMID: 19193784
- PMCID: PMC2663288
- DOI: 10.1128/JVI.01739-08
Human rhinovirus type 2 uncoating at the plasma membrane is not affected by a pH gradient but is affected by the membrane potential
Abstract
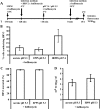
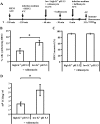
The minor receptor group human rhinovirus type 2 enters host cells by endocytosis via members of the low-density-lipoprotein receptor family. In late endosomes, it undergoes a conformational change solely induced by a pH of < or =5.6, resulting in RNA transfer across the endosomal membrane into the cytoplasm. To determine potential driving forces of this process, we investigated whether RNA penetration might depend on the pH gradient and/or the membrane potential between the acidic endosome lumen and the neutral cytoplasm. Since these parameters are difficult to assess in endosomes, we took advantage of the possibility of inducing structural changes, RNA release, and consequently infection from the plasma membrane. To manipulate the pH gradient, cell-bound virus was exposed to membrane-permeant or -impermeant acidic buffers at 4 degrees C, and this was followed by a shift to 34 degrees C in medium containing bafilomycin to prevent RNA release from endosomes. To manipulate the plasma membrane potential, similar experiments were carried out, but these included K(+) diffusion potentials in the presence of the K(+) ionophore valinomycin. We demonstrated that infection does not depend on a pH gradient but is enhanced by plasma membrane hyperpolarization compared to plasma membrane depolarization.
Figures



Similar articles
-
Conformational changes, plasma membrane penetration, and infection by human rhinovirus type 2: role of receptors and low pH.J Virol. 2003 May;77(9):5370-7. doi: 10.1128/jvi.77.9.5370-5377.2003. J Virol. 2003. PMID: 12692239 Free PMC article.
-
Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection.J Virol. 1998 Dec;72(12):9645-55. doi: 10.1128/JVI.72.12.9645-9655.1998. J Virol. 1998. PMID: 9811698 Free PMC article.
-
Uncoating of human rhinovirus serotype 2 from late endosomes.J Virol. 1994 Jun;68(6):3713-23. doi: 10.1128/JVI.68.6.3713-3723.1994. J Virol. 1994. PMID: 8189509 Free PMC article.
-
Uncoating of human rhinoviruses.Rev Med Virol. 2010 Sep;20(5):281-97. doi: 10.1002/rmv.654. Rev Med Virol. 2010. PMID: 20629045 Review.
-
Viral cell recognition and entry.Protein Sci. 1994 Oct;3(10):1712-25. doi: 10.1002/pro.5560031010. Protein Sci. 1994. PMID: 7849588 Free PMC article. Review.
Cited by
-
Liposomal nanocontainers as models for viral infection: monitoring viral genomic RNA transfer through lipid membranes.J Virol. 2011 Aug;85(16):8368-75. doi: 10.1128/JVI.00329-11. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680510 Free PMC article.
-
Inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 Replication by Hypertonic Saline Solution in Lung and Kidney Epithelial Cells.ACS Pharmacol Transl Sci. 2021 Sep 3;4(5):1514-1527. doi: 10.1021/acsptsci.1c00080. eCollection 2021 Oct 8. ACS Pharmacol Transl Sci. 2021. PMID: 34651104 Free PMC article.
-
Cullin-3 regulates late endosome maturation.Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):823-8. doi: 10.1073/pnas.1118744109. Epub 2012 Jan 4. Proc Natl Acad Sci U S A. 2012. PMID: 22219362 Free PMC article.
-
Valinomycin as a potential antiviral agent against coronaviruses: A review.Biomed J. 2020 Oct;43(5):414-423. doi: 10.1016/j.bj.2020.08.006. Epub 2020 Aug 11. Biomed J. 2020. PMID: 33012699 Free PMC article. Review.
-
Productive entry pathways of human rhinoviruses.Adv Virol. 2012;2012:826301. doi: 10.1155/2012/826301. Epub 2012 Nov 26. Adv Virol. 2012. PMID: 23227049 Free PMC article.
References
-
- Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 2763952-3962. - PubMed
-
- Borle, A. B., and J. Loveday. 1968. Effects of temperature, potassium, and calcium on the electrical potential difference in HeLa cells. Cancer Res. 282401-2405. - PubMed
-
- Brabec, M., D. Blaas, and R. Fuchs. 2006. Wortmannin delays transfer of human rhinovirus serotype 2 to late endocytic compartments. Biochem. Biophys. Res. Commun. 348741-749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

