Evaluation of different RNA extraction methods and storage conditions of dried plasma or blood spots for human immunodeficiency virus type 1 RNA quantification and PCR amplification for drug resistance testing
- PMID: 19193835
- PMCID: PMC2668360
- DOI: 10.1128/JCM.02255-08
Evaluation of different RNA extraction methods and storage conditions of dried plasma or blood spots for human immunodeficiency virus type 1 RNA quantification and PCR amplification for drug resistance testing
Abstract
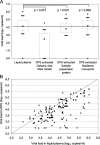
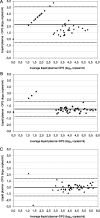
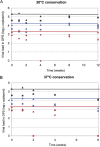
The development and validation of dried sample spots as a method of specimen collection are urgently needed in developing countries for monitoring of human immunodeficiency virus (HIV) infection. Our aim was to test some crucial steps in the use of dried spots, i.e., viral recovery and storage over time. Moreover, we investigated whether dried plasma and blood spots (DPS and DBS, respectively) give comparable viral load (VL) results. Four manual RNA extraction methods from commercial HIV type 1 (HIV-1) VL assays--a QIAamp minikit (Qiagen), the Abbott Molecular sample preparation system, the Nuclisens assay (bioMarieux), and High Pure viral nucleic acid kit (Roche Applied Science)--were compared for VL quantification and PCR amplification for genotypic drug resistance testing on dried spots from spiked plasma and residual samples from HIV-1 patients (n = 47; median VL, 4.13 log(10) copies/ml). RNA recovery from DPS was efficient using Nuclisens extraction (median difference, 0.03 log(10) copies/ml) and slightly underestimated using the Abbott Molecular sample preparation system (median difference, 0.35 log(10) copies/ml). PCR amplification results were in concordance. Measurements from DBS overestimated VL for plasma, with VL results showing <3.7 log(10) copies/ml. VL was stable for up to 3 months in spiked DPS stored at 20 degrees C but for only 1 month at 37 degrees C. A faster decline was observed in PCR efficiency: DPS could be stored for 1 week at 37 degrees C and for 1 month at 20 degrees C. In conclusion, the RNA extraction method is an important factor in obtaining reliable RNA quantification and PCR amplification of HIV-1 on DPS/DBS. DBS could be used as an alternative for DPS depending on HIV RNA cutoffs for virological failure. VL measurements remain stable over a longer period than do PCR amplification results.
Figures


Similar articles
-
Effect of storage conditions of dried plasma and blood spots on HIV-1 RNA quantification and PCR amplification for drug resistance genotyping.J Antimicrob Chemother. 2010 Aug;65(8):1562-6. doi: 10.1093/jac/dkq205. Epub 2010 Jun 11. J Antimicrob Chemother. 2010. PMID: 20542904
-
Rapid decline in the efficiency of HIV drug resistance genotyping from dried blood spots (DBS) and dried plasma spots (DPS) stored at 37 degrees C and high humidity.J Antimicrob Chemother. 2009 Jul;64(1):33-6. doi: 10.1093/jac/dkp150. Epub 2009 Apr 29. J Antimicrob Chemother. 2009. PMID: 19403653 Free PMC article.
-
Correlation between human immunodeficiency virus type 1 (HIV-1) RNA measurements obtained with dried blood spots and those obtained with plasma by use of Nuclisens EasyQ HIV-1 and Abbott RealTime HIV load tests.J Clin Microbiol. 2009 Apr;47(4):1031-6. doi: 10.1128/JCM.02099-08. Epub 2009 Feb 4. J Clin Microbiol. 2009. PMID: 19193847 Free PMC article.
-
Dried fluid spots for HIV type-1 viral load and resistance genotyping: a systematic review.Antivir Ther. 2009;14(5):619-29. Antivir Ther. 2009. PMID: 19704164
-
Measurement of HIV-1 viral load for drug resistance surveillance using dried blood spots: literature review and modeling of contribution of DNA and RNA.AIDS Rev. 2014 Jul-Sep;16(3):160-71. AIDS Rev. 2014. PMID: 25221990 Review.
Cited by
-
High rates of virological failure and drug resistance in perinatally HIV-1-infected children and adolescents receiving lifelong antiretroviral therapy in routine clinics in Togo.J Int AIDS Soc. 2016 Apr 27;19(1):20683. doi: 10.7448/IAS.19.1.20683. eCollection 2016. J Int AIDS Soc. 2016. PMID: 27125320 Free PMC article.
-
Implementation and Operational Research: Programmatic Feasibility of Dried Blood Spots for the Virological Follow-up of Patients on Antiretroviral Treatment in Nord Kivu, Democratic Republic of the Congo.J Acquir Immune Defic Syndr. 2016 Jan 1;71(1):e9-15. doi: 10.1097/QAI.0000000000000844. J Acquir Immune Defic Syndr. 2016. PMID: 26413848 Free PMC article.
-
Evaluation of quantification of HIV-1 RNA viral load in plasma and dried blood spots by use of the semiautomated Cobas Amplicor assay and the fully automated Cobas Ampliprep/TaqMan assay, version 2.0, in Kisumu, Kenya.J Clin Microbiol. 2013 Apr;51(4):1208-18. doi: 10.1128/JCM.03048-12. Epub 2013 Feb 6. J Clin Microbiol. 2013. PMID: 23390278 Free PMC article.
-
Field evaluation of dried blood spots for routine HIV-1 viral load and drug resistance monitoring in patients receiving antiretroviral therapy in Africa and Asia.J Clin Microbiol. 2014 Feb;52(2):578-86. doi: 10.1128/JCM.02860-13. Epub 2013 Dec 11. J Clin Microbiol. 2014. PMID: 24478491 Free PMC article.
-
Assessment of the direct quantitation of SARS-CoV-2 by droplet digital PCR.Sci Rep. 2020 Oct 30;10(1):18764. doi: 10.1038/s41598-020-75958-x. Sci Rep. 2020. PMID: 33127953 Free PMC article.
References
-
- Alvarez-Munoz, M. T., S. Zaragoza-Rodriguez, O. Rojas-Montes, G. Palacios-Saucedo, G. Vazquez-Rosales, A. Gomez-Delgado, J. Torres, and O. Munoz. 2005. High correlation of human immunodeficiency virus type-1 viral load measured in dried-blood spot samples and in plasma under different storage conditions. Arch. Med. Res. 36382-386. - PubMed
-
- Amellal, B., C. Katlama, and V. Calvez. 2007. Evaluation of the use of dried spots and of different storage conditions of plasma for HIV-1 RNA quantification. HIV Med. 8396-400. - PubMed
-
- Ayele, W., R. Schuurman, T. Messele, W. Dorigo-Zetsma, Y. Mengistu, J. Goudsmit, W. A. Paxton, M. P. de Baar, and G. Pollakis. 2007. Use of dried spots of whole blood, plasma, and mother's milk collected on filter paper for measurement of human immunodeficiency virus type 1 burden. J. Clin. Microbiol. 45891-896. - PMC - PubMed
-
- Barin, F., L. Meyer, R. Lancar, C. Deveau, M. Gharib, A. Laporte, J. C. Desenclos, and D. Costagliola. 2005. Development and validation of an immunoassay for identification of recent human immunodeficiency virus type 1 infections and its use on dried serum spots. J. Clin. Microbiol. 434441-4447. - PMC - PubMed
-
- Bertagnolio, S., I. Derdelinckx, M. Parker, J. Fitzgibbon, H. Fleury, M. Peeters, R. Schuurman, D. Pillay, L. Morris, A. Tanuri, G. M. Gershy-Damet, J. Nkengasong, C. F. Gilks, D. Sutherland, and P. Sandstrom. 2008. World Health Organization/HIVResNet drug resistance laboratory strategy. Antivir. Ther. 13(Suppl. 2)49-57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

