c-kit expression identifies cardiovascular precursors in the neonatal heart
- PMID: 19193854
- PMCID: PMC2644119
- DOI: 10.1073/pnas.0808920106
c-kit expression identifies cardiovascular precursors in the neonatal heart
Abstract
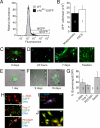
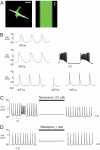
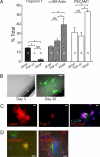
Directed differentiation of embryonic stem cells indicates that mesodermal lineages in the mammalian heart (cardiac, endothelial, and smooth muscle cells) develop from a common, multipotent cardiovascular precursor. To isolate and characterize the lineage potential of a resident pool of cardiovascular progenitor cells (CPcs), we developed BAC transgenic mice in which enhanced green fluorescent protein (EGFP) is placed under control of the c-kit locus (c-kit(BAC)-EGFP mice). Discrete c-kit-EGFP(+) cells were observed at different stages of differentiation in embryonic hearts, increasing in number to a maximum at about postnatal day (PN) 2; thereafter, EGFP(+) cells declined and were rarely observed in the adult heart. EGFP(+) cells purified from PN 0-5 hearts were nestin(+) and expanded in culture; 67% of cells were fluorescent after 9 days. Purified cells differentiated into endothelial, cardiac, and smooth muscle cells, and differentiation could be directed by specific growth factors. CPc-derived cardiac myocytes displayed rhythmic beating and action potentials characteristic of multiple cardiac cell types, similar to ES cell-derived cardiomyocytes. Single-cell dilution studies confirmed the potential of individual CPcs to form all 3 cardiovascular lineages. In adult hearts, cryoablation resulted in c-kit-EGFP(+) expression, peaking 7 days postcryolesion. Expression occurred in endothelial and smooth muscle cells in the revascularizing infarct, and in terminally differentiated cardiomyocytes in the border zone surrounding the infarct. Thus, c-kit expression marks CPc in the neonatal heart that are capable of directed differentiation in vitro; however, c-kit expression in cardiomyocytes in the adult heart after injury does not identify cardiac myogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
c-kit cardiac progenitor cells: what is their potential?Proc Natl Acad Sci U S A. 2009 Jul 14;106(28):E78; author reply E79. doi: 10.1073/pnas.0903261106. Epub 2009 Jul 1. Proc Natl Acad Sci U S A. 2009. PMID: 19570994 Free PMC article. No abstract available.
Similar articles
-
C-kit expression identifies cardiac precursor cells in neonatal mice.Methods Mol Biol. 2012;843:177-89. doi: 10.1007/978-1-61779-523-7_17. Methods Mol Biol. 2012. PMID: 22222532
-
Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart.Cell. 2006 Dec 15;127(6):1137-50. doi: 10.1016/j.cell.2006.10.028. Epub 2006 Nov 22. Cell. 2006. PMID: 17123591
-
c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart.Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):13380-5. doi: 10.1073/pnas.1208114109. Epub 2012 Jul 30. Proc Natl Acad Sci U S A. 2012. PMID: 22847442 Free PMC article.
-
The current status and future of cardiac stem/progenitor cell therapy for congenital heart defects from diabetic pregnancy.Pediatr Res. 2018 Jan;83(1-2):275-282. doi: 10.1038/pr.2017.259. Epub 2017 Nov 15. Pediatr Res. 2018. PMID: 29016556 Free PMC article. Review.
-
"String theory" of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results.Circ Res. 2015 Mar 27;116(7):1216-30. doi: 10.1161/CIRCRESAHA.116.305557. Circ Res. 2015. PMID: 25814683 Free PMC article. Review.
Cited by
-
Regenerating the ailing heart: Stem cell therapies for hypoplastic left heart syndrome.Ann Pediatr Cardiol. 2024 Mar-Apr;17(2):124-131. doi: 10.4103/apc.apc_24_24. Epub 2024 Jul 20. Ann Pediatr Cardiol. 2024. PMID: 39184114 Free PMC article. Review.
-
Partially Digested Adult Cardiac Extracellular Matrix Promotes Cardiomyocyte Proliferation In Vitro.Adv Healthc Mater. 2015 Jul 15;4(10):1545-54. doi: 10.1002/adhm.201500035. Epub 2015 May 18. Adv Healthc Mater. 2015. PMID: 25988681 Free PMC article.
-
Stem cells in thoracic aortic aneurysms and dissections: potential contributors to aortic repair.Ann Thorac Surg. 2012 May;93(5):1524-33. doi: 10.1016/j.athoracsur.2012.01.063. Epub 2012 Mar 20. Ann Thorac Surg. 2012. PMID: 22440369 Free PMC article.
-
Progenitor/stem cell transplantation for repair of myocardial infarction: Hype or hope?Ann Palliat Med. 2012;1(1):65-77. doi: 10.3978/j.issn.2224-5820.2012.04.01. Ann Palliat Med. 2012. PMID: 22833840 Free PMC article.
-
Stem cell therapies in cardiac diseases: Current status and future possibilities.World J Stem Cells. 2021 Sep 26;13(9):1231-1247. doi: 10.4252/wjsc.v13.i9.1231. World J Stem Cells. 2021. PMID: 34630860 Free PMC article. Review.
References
-
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. - PubMed
-
- Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. - PubMed
-
- Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. - PubMed
-
- Wu SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. - PubMed
-
- Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

