Control and function of the homeostatic sleep response by adenosine A1 receptors
- PMID: 19193874
- PMCID: PMC2754857
- DOI: 10.1523/JNEUROSCI.2942-08.2009
Control and function of the homeostatic sleep response by adenosine A1 receptors
Abstract
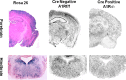
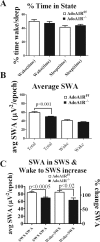
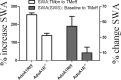
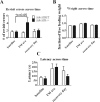
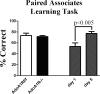
During sleep, the mammalian CNS undergoes widespread, synchronized slow-wave activity (SWA) that directly varies with previous waking duration (Borbély, 1982; Dijk et al., 1990). When sleep is restricted, an enhanced SWA response follows in the next sleep period. The enhancement of SWA is associated with improved cognitive performance (Huber et al., 2004), but it is unclear either how the SWA is enhanced or whether SWA is needed to maintain normal cognitive performance. A conditional, CNS knock-out of the adenosine receptor, AdoA(1)R gene, shows selective attenuation of the SWA rebound response to restricted sleep, but sleep duration is not affected. During sleep restriction, wild phenotype animals express a rebound SWA response and maintain cognitive performance in a working memory task. However, the knock-out animals not only show a reduced rebound SWA response but they also fail to maintain normal cognitive function, although this function is normal when sleep is not restricted. Thus, AdoA(1)R activation is needed for normal rebound SWA, and when the SWA rebound is reduced, there is a failure to maintain working memory function, suggesting a functional role for SWA homeostasis.
Figures




References
-
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. - PubMed
-
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. - PubMed
-
- Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
