Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex
- PMID: 19193877
- PMCID: PMC6666091
- DOI: 10.1523/JNEUROSCI.5446-08.2009
Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex
Abstract
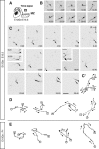
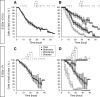
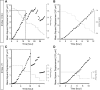
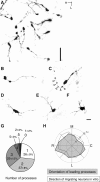
Migrating neurons are thought to travel from their origin near the ventricle to distant territories along stereotypical pathways by detecting environmental cues in the extracellular milieu. Here, we report a novel mode of neuronal migration that challenges this view. We performed long-term, time-lapse imaging of medial ganglionic eminence (MGE)-derived cortical interneurons tangentially migrating in the marginal zone (MZ) in flat-mount cortices. We find that they exhibit a diverse range of behaviors in terms of the rate and direction of migration. Curiously, a predominant population of these neurons repeatedly changes its direction of migration in an unpredictable manner. Trajectories of migration vary from one neuron to another. The migration of individual cells lasts for long periods, sometimes up to 2 d. Theoretical analyses reveal that these behaviors can be modeled by a random walk. Furthermore, MZ cells migrate from the cortical subventricular zone to the cortical plate, transiently accumulating in the MZ. These results suggest that MGE-derived cortical interneurons, once arriving at the MZ, are released from regulation by guidance cues and initiate random walk movement, which potentially contributes to their dispersion throughout the cortex.
Figures



Comment in
-
The wanderlust of newborn neocortical interneurons.J Neurosci. 2009 Jun 3;29(22):7114-5. doi: 10.1523/JNEUROSCI.1624-09.2009. J Neurosci. 2009. PMID: 19494133 Free PMC article. Review. No abstract available.
Similar articles
-
Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex.Development. 2006 Jun;133(11):2167-76. doi: 10.1242/dev.02382. Epub 2006 May 3. Development. 2006. PMID: 16672340
-
Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins.Development. 2003 Dec;130(23):5803-13. doi: 10.1242/dev.00825. Epub 2003 Oct 8. Development. 2003. PMID: 14534141
-
Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone.J Neurosci. 2006 Dec 20;26(51):13273-8. doi: 10.1523/JNEUROSCI.4162-06.2006. J Neurosci. 2006. PMID: 17182777 Free PMC article.
-
Intracortical multidirectional migration of cortical interneurons.Novartis Found Symp. 2007;288:116-25; discussion 125-9, 276-81. Novartis Found Symp. 2007. PMID: 18494255 Review.
-
Molecules and mechanisms involved in the generation and migration of cortical interneurons.ASN Neuro. 2010 Mar 31;2(2):e00031. doi: 10.1042/AN20090053. ASN Neuro. 2010. PMID: 20360946 Free PMC article. Review.
Cited by
-
Cortical GABAergic interneurons transiently assume a sea urchin-like nonpolarized shape before axon initiation.J Neurosci. 2010 Nov 10;30(45):15221-7. doi: 10.1523/JNEUROSCI.1527-10.2010. J Neurosci. 2010. PMID: 21068327 Free PMC article.
-
Neurotransmitter signaling regulates distinct phases of multimodal human interneuron migration.EMBO J. 2021 Dec 1;40(23):e108714. doi: 10.15252/embj.2021108714. Epub 2021 Oct 18. EMBO J. 2021. PMID: 34661293 Free PMC article.
-
The Epigenome in Neurodevelopmental Disorders.Front Neurosci. 2021 Nov 3;15:776809. doi: 10.3389/fnins.2021.776809. eCollection 2021. Front Neurosci. 2021. PMID: 34803599 Free PMC article. Review.
-
Characterization and Stage-Dependent Lineage Analysis of Intermediate Progenitors of Cortical GABAergic Interneurons.Front Neurosci. 2021 Jul 8;15:607908. doi: 10.3389/fnins.2021.607908. eCollection 2021. Front Neurosci. 2021. PMID: 34305510 Free PMC article.
-
Four distinct phases of basket/stellate cell migration after entering their final destination (the molecular layer) in the developing cerebellum.Dev Biol. 2009 Aug 15;332(2):309-24. doi: 10.1016/j.ydbio.2009.05.575. Epub 2009 Jun 3. Dev Biol. 2009. PMID: 19500566 Free PMC article.
References
-
- Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. - PubMed
-
- Berg HC. Random walks in biology. Princeton, NJ: Princeton UP; 1993.
-
- Bloch-Gallego E, Causeret F, Ezan F, Backer S, Hidalgo-Sanchez M. Development of precerebellar nuclei: instructive factors and intracellular mediators in neuronal migration, survival and axon pathfinding. Brain Res Brain Res Rev. 2005;49:253–266. - PubMed
-
- Borrell V, Marín O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
