Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells
- PMID: 19193893
- PMCID: PMC2650785
- DOI: 10.1523/JNEUROSCI.4240-08.2009
Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells
Abstract
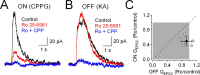
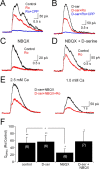
NMDA receptors (NMDARs) are tetrameric protein complexes usually comprising two NR1 and two NR2 subunits. Different combinations of four potential NR2 subunits (NR2A-D) confer diversity in developmental expression, subsynaptic localization, and functional characteristics, including affinity for neurotransmitter. NR2B-containing NMDARs, for example, exhibit relatively high affinity both for glutamate and the coagonist glycine. Although multiple NMDAR subtypes can colocalize at individual synapses, particular subtypes often mediate inputs from distinct functional pathways. In retinal ganglion cells (RGCs), NMDARs contribute to synaptic responses elicited by light stimulus onset ("ON") and offset ("OFF"), but roles for particular NMDAR subtypes, and potential segregation between the ON and OFF pathways, have not been explored. Moreover, elements in the retinal circuitry release two different NMDAR coagonists, glycine and d-serine, but the effects of endogenous coagonist release on the relative contribution of different NMDAR subtypes are unclear. Here, we show that coagonist release within the retina modulates the relative contribution of different NMDARs in the ON pathway of the rat retina. By pharmacologically stimulating functional pathways independently in acute slices and recording synaptic responses in RGCs, we show that ON inputs, but not OFF inputs, are mediated in part by NMDARs exhibiting NR2B-like pharmacology. Furthermore, suppressing release of NMDAR coagonist reduces NMDAR activation at ON synapses and increases the relative contribution of these putative NR2B-containing receptors. These results demonstrate direct evidence for evoked coagonist release onto NMDARs and indicate that modulating coagonist release may regulate the relative activation of different NMDAR subtypes in the ON pathway.
Figures






Similar articles
-
Subunit- and pathway-specific localization of NMDA receptors and scaffolding proteins at ganglion cell synapses in rat retina.J Neurosci. 2009 Apr 1;29(13):4274-86. doi: 10.1523/JNEUROSCI.5602-08.2009. J Neurosci. 2009. PMID: 19339621 Free PMC article.
-
Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina.J Neurosci. 2002 Mar 15;22(6):2165-73. doi: 10.1523/JNEUROSCI.22-06-02165.2002. J Neurosci. 2002. PMID: 11896156 Free PMC article.
-
AMPA receptor-dependent, light-evoked D-serine release acts on retinal ganglion cell NMDA receptors.J Neurophysiol. 2012 Aug;108(4):1044-51. doi: 10.1152/jn.00264.2012. Epub 2012 May 16. J Neurophysiol. 2012. PMID: 22592312 Free PMC article.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review.
Cited by
-
An intrinsic neural oscillator in the degenerating mouse retina.J Neurosci. 2011 Mar 30;31(13):5000-12. doi: 10.1523/JNEUROSCI.5800-10.2011. J Neurosci. 2011. PMID: 21451038 Free PMC article.
-
Dynamic regulation of D-serine release in the vertebrate retina.J Physiol. 2015 Feb 15;593(4):843-56. doi: 10.1113/jphysiol.2014.283432. Epub 2015 Jan 7. J Physiol. 2015. PMID: 25480802 Free PMC article.
-
Light-evoked NMDA receptor-mediated currents are reduced by blocking D-serine synthesis in the salamander retina.Neuroreport. 2010 Mar 10;21(4):239-44. doi: 10.1097/WNR.0b013e32833313b7. Neuroreport. 2010. PMID: 20101193 Free PMC article.
-
The mode of retinal presynaptic inhibition switches with light intensity.J Neurosci. 2012 Mar 28;32(13):4360-71. doi: 10.1523/JNEUROSCI.5645-11.2012. J Neurosci. 2012. PMID: 22457487 Free PMC article.
-
Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2393-E2402. doi: 10.1073/pnas.1714888115. Epub 2018 Feb 20. Proc Natl Acad Sci U S A. 2018. PMID: 29463759 Free PMC article.
References
-
- Ahmadi S, Muth-Selbach U, Lauterbach A, Lipfert P, Neuhuber WL, Zeilhofer HU. Facilitation of spinal NMDA receptor currents by spillover of synaptically released glycine. Science. 2003;300:2094–2097. - PubMed
-
- Bashir ZI, Tam B, Collingridge GL. Activation of the glycine site in the NMDA receptor is necessary for the induction of LTP. Neurosci Lett. 1990;108:261–266. - PubMed
-
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. - PubMed
-
- Brandstätter JH, Hartveit E, Sassoè-Pognetto M, Wässle H. Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci. 1994;6:1100–1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
