Rab11a and HSP90 regulate recycling of extracellular alpha-synuclein
- PMID: 19193894
- PMCID: PMC3241981
- DOI: 10.1523/JNEUROSCI.6202-08.2009
Rab11a and HSP90 regulate recycling of extracellular alpha-synuclein
Abstract
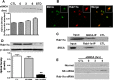
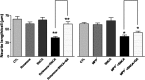
Growing evidence suggests that extracellular alpha-synuclein (eSNCA) may play an important role in the pathogenesis of Parkinson's disease (PD) and related synucleinopathies by producing neurotoxicity directly or via activation of glia. However, the mechanisms involved in the trafficking of eSNCA in neurons and/or glia remain unclear. Here, we demonstrated that eSNCA could be resecreted out of neurons via a process modulated by a recycling endosome regulator rab11a in addition to being degraded by an endosome-lysosome system. A quantitative proteomic analysis also revealed numerous proteins through which rab11a might execute its function. One of the candidate proteins, heat shock protein 90 (HSP90), was validated to be interacting with rab11a. Furthermore, geldanamycin, an HSP90 inhibitor, not only prevented resecretion of eSNCA but also attenuated neurotoxicity induced by eSNCA.
Figures

Similar articles
-
Identification of ciliary neurotrophic factor receptor alpha as a mediator of neurotoxicity induced by alpha-synuclein.Proteomics. 2010 Jun;10(11):2138-50. doi: 10.1002/pmic.200900745. Proteomics. 2010. PMID: 20340160 Free PMC article.
-
HSP90 drives the Rab11a-mediated vesicular transport of the cell surface receptors in osteoclasts.Cell Biochem Funct. 2022 Dec;40(8):838-855. doi: 10.1002/cbf.3745. Epub 2022 Sep 16. Cell Biochem Funct. 2022. PMID: 36111708
-
Membrane binding, internalization, and sorting of alpha-synuclein in the cell.Acta Neuropathol Commun. 2018 Aug 14;6(1):79. doi: 10.1186/s40478-018-0578-1. Acta Neuropathol Commun. 2018. PMID: 30107856 Free PMC article.
-
The LRRK2-RAB axis in regulation of vesicle trafficking and α-synuclein propagation.Biochim Biophys Acta Mol Basis Dis. 2020 Mar 1;1866(3):165632. doi: 10.1016/j.bbadis.2019.165632. Epub 2019 Dec 6. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31812666 Review.
-
α-Synuclein Trafficking in Parkinson's Disease: Insights From Fly and Mouse Models.ASN Neuro. 2018 Jan-Dec;10:1759091418812587. doi: 10.1177/1759091418812587. ASN Neuro. 2018. PMID: 30482039 Free PMC article. Review.
Cited by
-
The GTPase Rab27b regulates the release, autophagic clearance, and toxicity of α-synuclein.J Biol Chem. 2020 Jun 5;295(23):8005-8016. doi: 10.1074/jbc.RA120.013337. Epub 2020 Apr 29. J Biol Chem. 2020. PMID: 32350025 Free PMC article.
-
Alpha-synuclein Toxicity in the Early Secretory Pathway: How It Drives Neurodegeneration in Parkinsons Disease.Front Neurosci. 2015 Nov 12;9:433. doi: 10.3389/fnins.2015.00433. eCollection 2015. Front Neurosci. 2015. PMID: 26617485 Free PMC article. Review.
-
The Contribution of α-Synuclein Spreading to Parkinson's Disease Synaptopathy.Neural Plast. 2017;2017:5012129. doi: 10.1155/2017/5012129. Epub 2017 Jan 3. Neural Plast. 2017. PMID: 28133550 Free PMC article. Review.
-
Induction of intracellular tau aggregation is promoted by α-synuclein seeds and provides novel insights into the hyperphosphorylation of tau.J Neurosci. 2011 May 25;31(21):7604-18. doi: 10.1523/JNEUROSCI.0297-11.2011. J Neurosci. 2011. PMID: 21613474 Free PMC article.
-
A Critical Assessment of Exosomes in the Pathogenesis and Stratification of Parkinson's Disease.J Parkinsons Dis. 2017;7(4):569-576. doi: 10.3233/JPD-171176. J Parkinsons Dis. 2017. PMID: 28922170 Free PMC article. Review.
References
-
- Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. - PubMed
-
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. - PMC - PubMed
-
- El-Agnaf OM, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, Pessi A, Neill D, Wallace A. Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett. 1998;440:71–75. - PubMed
-
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, Cookson MR, Hardy J, Allsop D. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
