Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin
- PMID: 19193897
- PMCID: PMC2751610
- DOI: 10.1523/JNEUROSCI.5147-08.2009
Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin
Abstract
Hypocretin/orexin, produced by a group of neurons in the lateral hypothalamus/perifornical area, enhances cognitive arousal and also may play a crucial role in modulating the neuroendocrine system. How hypocretin modulates the endocrine system remains an open question. Hypocretin cells innervate the mediobasal hypothalamus where they can potentially influence the activity of specific cell populations within the arcuate nucleus. Here, we examine whether hypocretin modulates the median eminence-projecting proopiomelanocortin (POMC) neurons identified by selective green fluorescent protein expression and antidromic stimulation or retrograde Evans blue dye tracing in transgenic mice. We find that POMC neurons, in general, and, in addition, those that project their axons to the median eminence, were robustly activated by hypocretin in a dose-dependent manner. These excitatory actions included a threefold increase in spike frequency and direct membrane depolarization of up to 22 mV (mean, 17.9+/-7.2 mV). Direct postsynaptic depolarization was decreased at more positive membrane potentials, inhibited by the sodium-calcium exchanger antagonist KB-R7943, and reduced by lowering the bath temperature, or by buffering the postsynaptic calcium with BAPTA, suggesting that the primary mechanism for hypocretin-mediated excitation is the activation of the sodium-calcium exchanger. Hypocretin also enhanced excitatory inputs to POMC cells via a presynaptic mechanism and indirectly increased the release of GABA onto these cells in a spike-dependent manner. However, these synaptic actions were not necessary to cause postsynaptic membrane depolarization and spiking. Thus, in contrast to previous suggestions that hypocretin inhibited POMC cells, our results demonstrate robust direct excitation of POMC neurons by hypocretin.
Figures

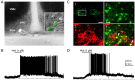
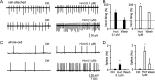



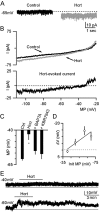
Similar articles
-
Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences.J Neurophysiol. 2011 Sep;106(3):1191-202. doi: 10.1152/jn.00740.2010. Epub 2011 Jun 8. J Neurophysiol. 2011. PMID: 21653710 Free PMC article.
-
Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system.J Neurosci. 2004 Oct 6;24(40):8741-51. doi: 10.1523/JNEUROSCI.2268-04.2004. J Neurosci. 2004. PMID: 15470140 Free PMC article.
-
Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons.J Neurosci. 2005 Jan 5;25(1):173-83. doi: 10.1523/JNEUROSCI.4015-04.2005. J Neurosci. 2005. PMID: 15634779 Free PMC article.
-
Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction.Brain Res. 2010 Feb 16;1314:74-90. doi: 10.1016/j.brainres.2009.09.106. Epub 2009 Oct 6. Brain Res. 2010. PMID: 19815001 Free PMC article. Review.
-
The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function.Front Neuroendocrinol. 2003 Jul;24(3):141-50. doi: 10.1016/s0091-3022(03)00028-1. Front Neuroendocrinol. 2003. PMID: 14596809 Review.
Cited by
-
Electrophysiological analysis of circuits controlling energy homeostasis.Mol Neurobiol. 2012 Apr;45(2):258-78. doi: 10.1007/s12035-012-8241-5. Epub 2012 Feb 14. Mol Neurobiol. 2012. PMID: 22331510 Review.
-
Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake.PLoS Biol. 2018 Apr 24;16(4):e2004399. doi: 10.1371/journal.pbio.2004399. eCollection 2018 Apr. PLoS Biol. 2018. PMID: 29689050 Free PMC article.
-
Neuroactive peptides as putative mediators of antiepileptic ketogenic diets.Front Neurol. 2014 Apr 29;5:63. doi: 10.3389/fneur.2014.00063. eCollection 2014. Front Neurol. 2014. PMID: 24808888 Free PMC article. Review.
-
Depolarization-induced suppression of spontaneous release in the avian midbrain.J Neurosci. 2011 Mar 9;31(10):3602-9. doi: 10.1523/JNEUROSCI.6388-10.2011. J Neurosci. 2011. PMID: 21389216 Free PMC article.
-
Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences.J Neurophysiol. 2011 Sep;106(3):1191-202. doi: 10.1152/jn.00740.2010. Epub 2011 Jun 8. J Neurophysiol. 2011. PMID: 21653710 Free PMC article.
References
-
- Al-Barzanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS. Central orexin-A activates the hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurons in conscious rats. J Neuroendocrinol. 2001;13:421–424. - PubMed
-
- Bäckberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. - PubMed
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. - PubMed
-
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
