Positron emission tomography imaging demonstrates correlation between behavioral recovery and correction of dopamine neurotransmission after gene therapy
- PMID: 19193901
- PMCID: PMC6666088
- DOI: 10.1523/JNEUROSCI.4491-08.2009
Positron emission tomography imaging demonstrates correlation between behavioral recovery and correction of dopamine neurotransmission after gene therapy
Abstract
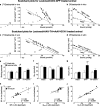
In vivo gene transfer using viral vectors is an emerging therapy for neurodegenerative diseases with a clinical impact recently demonstrated in Parkinson's disease patients. Recombinant adeno-associated viral (rAAV) vectors, in particular, provide an excellent tool for long-term expression of therapeutic genes in the brain. Here we used the [(11)C]raclopride [(S)-(-)-3,5-dichloro-N-((1-ethyl-2-pyrrolidinyl)methyl)-2-hydroxy-6-methoxybenzamide] micro-positron emission tomography (PET) technique to demonstrate that delivery of the tyrosine hydroxylase (TH) and GTP cyclohydrolase 1 (GCH1) enzymes using an rAAV5 vector normalizes the increased [(11)C]raclopride binding in hemiparkinsonian rats. Importantly, we show in vivo by microPET imaging and postmortem by classical binding assays performed in the very same animals that the changes in [(11)C]raclopride after viral vector-based enzyme replacement therapy is attributable to a decrease in the affinity of the tracer binding to the D(2) receptors, providing evidence for reconstitution of a functional pool of endogenous dopamine in the striatum. Moreover, the extent of the normalization in this non-invasive imaging measure was highly correlated with the functional recovery in motor behavior. The PET imaging protocol used in this study is fully adaptable to humans and thus can serve as an in vivo imaging technique to follow TH + GCH1 gene therapy in PD patients and provide an additional objective measure to a potential clinical trial using rAAV vectors to deliver l-3,4-dihydroxyphenylanaline in the brain.
Figures




References
-
- Antonini A, Leenders KL, Vontobel P, Maguire RP, Missimer J, Psylla M, Günther I. Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson's disease. Brain. 1997;120:2187–2195. - PubMed
-
- Carlsson T, Winkler C, Burger C, Muzyczka N, Mandel RJ, Cenci A, Björklund A, Kirik D. Reversal of dyskinesias in an animal model of Parkinson's disease by continuous l-DOPA delivery using rAAV vectors. Brain. 2005;128:559–569. - PubMed
-
- Carlsson T, Björklund T, Kirik D. Restoration of the striatal dopamine synthesis for Parkinson's disease: viral vector-mediated enzyme replacement strategy. Curr Gene Ther. 2007;7:109–120. - PubMed
-
- Chefer SI, Kimes AS, Matochik JA, Horti AG, Kurian V, Shumway D, Domino EF, London ED, Mukhin AG. Estimation of D2-like receptor occupancy by dopamine in the putamen of hemiparkinsonian monkeys. Neuropsychopharmacology. 2008;33:270–278. - PubMed
-
- Chritin M, Savasta M, Mennicken F, Bal A, Abrous DN, Le Moal M, Feuerstein C, Herman JP. Intrastriatal dopamine-rich implants reverse the increase of dopamine D2 receptor mRNA levels caused by lesion of the nigrostriatal pathway: a quantitative in situ hybridization study. Eur J Neurosci. 1992;4:663–672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
